��Ŀ����
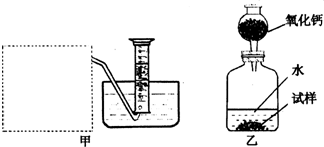
�Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���塣Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
�������Ѿ�֪����Na2O��H2O===2NaOH�������Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ��2Na2O2��2H2O=4NaOH+ ����������������������������������������������������
�Ƹ���ȤС���ͬѧ����˼�������װ�ã���װ�������߷�����Ϊ���巢��װ�ã�����ΪӦ��ʵ������ȡ ��������װ����ͬ������ȤС��ѡ�����ַ����ռ��������������������������������������Ϊ�����������������������ռ���
����װ�������ⶨ�����������������������������
�ȼ�װ�������ⶨ���������������������״���£�����֪��������������Ҫ�����Na2O2�Ĵ��ȣ�����Ҫ֪������������������������
A. �����ڱ�״���µ��ܶ� B. ��Ӧװ���м���ˮ������ C. ��Ӧ����Һ������
�������Ѿ�֪����Na2O��H2O===2NaOH�������Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ��2Na2O2��2H2O=4NaOH+ ����������������������������������������������������
�Ƹ���ȤС���ͬѧ����˼�������װ�ã���װ�������߷�����Ϊ���巢��װ�ã�����ΪӦ��ʵ������ȡ ��������װ����ͬ������ȤС��ѡ�����ַ����ռ��������������������������������������Ϊ�����������������������ռ���
����װ�������ⶨ�����������������������������
�ȼ�װ�������ⶨ���������������������״���£�����֪��������������Ҫ�����Na2O2�Ĵ��ȣ�����Ҫ֪������������������������
A. �����ڱ�״���µ��ܶ� B. ��Ӧװ���м���ˮ������ C. ��Ӧ����Һ������
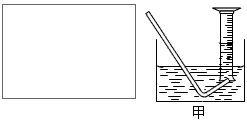
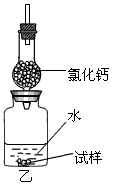
��O2�������ǵ�ľ�������������ܷ�ʹ�临ȼ
�ƶ�����̼��������������������ˮ�������ſ���
������
�������A
�ƶ�����̼��������������������ˮ�������ſ���
������
�������A

��ϰ��ϵ�д�
�����Ŀ

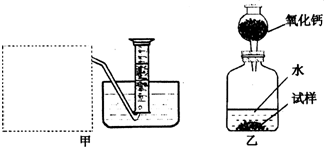 �Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
�Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺