��Ŀ����
ˮ�೧�����ң�Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ������������������Ϊ7.3%��ϡ�������ձ�������Ϊ50g���з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���
| ��Ӧǰ | ��Ӧǰ | ��Ӧ�� | |
| ʵ �� �� �� | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ��л��������� |
| 150g | 12g | 157.6g |
��2��������Ӧ�ķ���ʽΪ��______��
��3�����12g��ʯ��ʯ��̼���������X���ı���ʽΪ��______��
��4����ʯ��ʯ��Ʒ��̼��Ƶ����������ǣ�______��
��5����Ӧ��������Һ�м���116.4gˮ����������Һ�����ʵ�����������______��
��6����ˮ�೧��120g��ʯ��ʯ���Ƶú����ʵ���ʯ�ҵ�����Ϊ______g�����������һλС����
�⣺��1����Һ��ϡ��ǰ��������������䣬
��Ҫ36.5%��Ũ���������Ϊ��y
36.5%��y=7.3%����150-50��
y=20g
��2��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ʴ�Ϊ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3�����������غ㶨�ɿ�֪�����ɵĶ�����̼�������ǣ�150+12-157.6=4.4g��
������4.4�˵Ķ�����̼��Ҫ̼���Ϊx�������Ȼ���Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 111 44
x z 4.4g
 =
=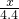 =
=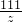
x=10g
z=11.1g
����̼��Ƶ���������Ϊ�� 100%=83.3%��
100%=83.3%��
��Ӧ�����Һ����Ϊ��107.6g-2g+116.4=222�ˣ�������������Ϊ�� 100%=5%��
100%=5%��
����Ƶö�����̼������Ϊm��
CaCO3 CaO+CO2��
CaO+CO2��
100 44
120��83.3% m
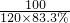 =
=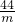
m=44g��
�������ɵĺ����ʵ��������ǣ�120-44=76�ˣ�
�ʴ�Ϊ����1��20��
��2��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3�� =
=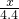 ��
��
��4��83.3%
��5��5%
��6��76��
��������1������ϡ��ǰ�����������������з�����
��2���������еķ�Ӧԭ����д����ʽ��
��3�����ݷ���ʽ�еĵ�����ϵ�б���ʽ��
��4���������ɵĶ�����̼��Ϸ���ʽ�еĵ�����ϵ���������̼��Ƶ���������������������
��5���������е������ҳ���Һ�����������������н��
��6������̼��Ƶ����������ͷ���ʽ�г�����ʽ���
�������ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ�����е�֪ʶ���з������
��Ҫ36.5%��Ũ���������Ϊ��y
36.5%��y=7.3%����150-50��
y=20g
��2��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ʴ�Ϊ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3�����������غ㶨�ɿ�֪�����ɵĶ�����̼�������ǣ�150+12-157.6=4.4g��
������4.4�˵Ķ�����̼��Ҫ̼���Ϊx�������Ȼ���Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 111 44
x z 4.4g
 =
=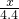 =
=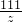
x=10g
z=11.1g
����̼��Ƶ���������Ϊ��
 100%=83.3%��
100%=83.3%����Ӧ�����Һ����Ϊ��107.6g-2g+116.4=222�ˣ�������������Ϊ��
 100%=5%��
100%=5%������Ƶö�����̼������Ϊm��
CaCO3
 CaO+CO2��
CaO+CO2��100 44
120��83.3% m
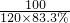 =
=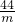
m=44g��
�������ɵĺ����ʵ��������ǣ�120-44=76�ˣ�
�ʴ�Ϊ����1��20��
��2��CaCO3+2HCl�TCaCl2+H2O+CO2����
��3��
 =
=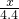 ��
����4��83.3%
��5��5%
��6��76��
��������1������ϡ��ǰ�����������������з�����
��2���������еķ�Ӧԭ����д����ʽ��
��3�����ݷ���ʽ�еĵ�����ϵ�б���ʽ��
��4���������ɵĶ�����̼��Ϸ���ʽ�еĵ�����ϵ���������̼��Ƶ���������������������
��5���������е������ҳ���Һ�����������������н��
��6������̼��Ƶ����������ͷ���ʽ�г�����ʽ���
�������ڽ������ʱ�����ȷ������п�������⣬Ȼ����ѧ����֪ʶ�����е�֪ʶ���з������

��ϰ��ϵ�д�
�����Ŀ
ijˮ�೧�����ң�Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ������ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���
��1�����������غ㶨�ɿ�֪����Ӧ�����ɶ�����̼������Ϊ g��
��2�����ʯ��ʯ��̼��Ƶ�����������
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ �� �� �� |
�ձ���ϡ���� ������ |
ʯ��ʯ��Ʒ ������ |
�ձ������л� ��������� |
| 150g | 12g | 157.6g | |
��2�����ʯ��ʯ��̼��Ƶ�����������
����ˮ�೧�����ң�Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ������ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���
��1�����������غ㶨�ɿ�֪����Ӧ�����ɶ�����̼������Ϊ g��
��2�����ʯ��ʯ��̼��Ƶ�����������
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ������ | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ������л��������� |
| 160g | 12g | 167.6g | |
��2�����ʯ��ʯ��̼��Ƶ�����������