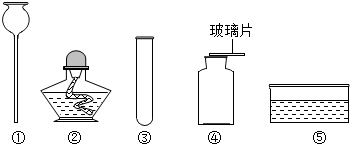
��Ŀ����
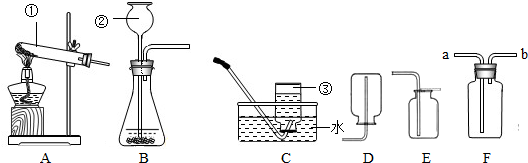
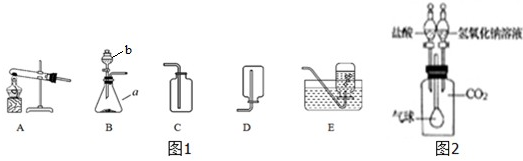
��ͼ��С��ͬѧ��Ƶ��ù���������Һ��������̻����ȡ����װ�ã�
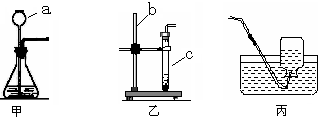
��1��ͼ��abc��ָ���������ƣ�
a______��b______��c______��
��2������װ�ñȽϣ���װ�õ��ŵ�______��μ��������ԣ�______��
��3���������Լ���ȡ�����Ļ�ѧ��Ӧ����ʽ��______��
��4������ռ�����������������ԭ����______������ţ�
A�����ܿ�һ�����ݾ������ռ�����B��װ��©��������C������ƿ��ûװ��ˮ���ռ�
��5������ø����������ȡ�������ɰ�װ���ҵ��Թܰڷ�λ�������Ķ���ͬʱ������ϵ�һ�ֲ�������������______���ø��������ȡ�����Ļ�ѧ����ʽ�ǣ�______
��6����������װ�õ��ռ�������Ϊ______��������ʵ������ȡCO2����
д����ȡCO2�ķ�Ӧ����ʽ��______��֤�ռ���CO2�ķ����ǣ�______
��7�����ֻ��ˮ�ۣ�����Ƭ�ͼ���ƿ��װ��CO2���������֤������̼����ˮ______��

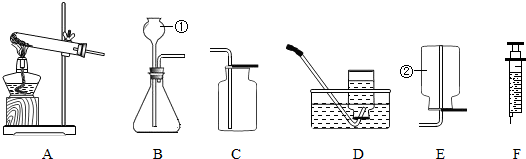
��1��ͼ��abc��ָ���������ƣ�
a______��b______��c______��
��2������װ�ñȽϣ���װ�õ��ŵ�______��μ��������ԣ�______��
��3���������Լ���ȡ�����Ļ�ѧ��Ӧ����ʽ��______��
��4������ռ�����������������ԭ����______������ţ�
A�����ܿ�һ�����ݾ������ռ�����B��װ��©��������C������ƿ��ûװ��ˮ���ռ�
��5������ø����������ȡ�������ɰ�װ���ҵ��Թܰڷ�λ�������Ķ���ͬʱ������ϵ�һ�ֲ�������������______���ø��������ȡ�����Ļ�ѧ����ʽ�ǣ�______
��6����������װ�õ��ռ�������Ϊ______��������ʵ������ȡCO2����
д����ȡCO2�ķ�Ӧ����ʽ��______��֤�ռ���CO2�ķ����ǣ�______
��7�����ֻ��ˮ�ۣ�����Ƭ�ͼ���ƿ��װ��CO2���������֤������̼����ˮ______��
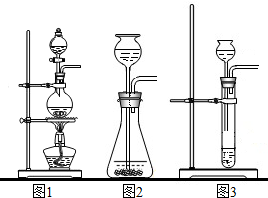
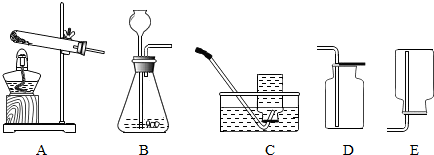
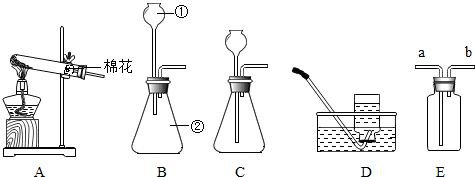
��1��ͼ����������������Ϊ������©��������̨���Թܣ�
��2����װ�ÿ��ڵ����ܵ���Ƥ���ϼ��ϼ��ӣ�ͨ�����ӵĿ�����ʱ���Ʒ�Ӧ�ķ�����ֹͣ������װ�õ������Կ����ü��Ӽ�ס�������е���Ƥ�ܣ�����A�м���ˮ���γ�һ��ˮ�������ã����۲쵽һ��ʱ��ˮ��Һ�治�½���˵��װ�����������ã�
��3������������Һ�ڶ������̵Ĵ�����֮�²�����ˮ���������÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O2
2H2O+O2����
��4��������ˮ���ռ�����ʱ�������ܿ�һ�����ݾ������ռ������ܻ�����Թ��ڵĿ�����ʹ�ռ�������������װ��©���ᵼ���ռ����������ᵼ����������������ƿ��ûװ��ˮ���ռ����������������ռ�������������
��5���ø��������ȡ��������ȣ��ʻ������Ӿƾ��ƣ���Ӧ�ķ���ʽ�ǣ�2KMnO4
K2MnO4+MnO2+O2����
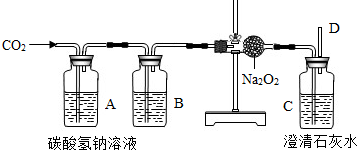
��6��������̼������ˮ����������ˮ���ռ������ܶȱȿ������������ſ������ռ���ʵ������ȡ������̼�ô���ʯ��ϡ���ᣬ��Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2��������������̼�ɽ�ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��۲�ľ���Ƿ�Ϩ������жϣ�
��7��Ҫ��֤������̼����ˮ���ɽ�װ��CO2�ļ���ƿ�����ڷ���ˮ��ˮ���У��۲켯��ƿ��ˮ���Ƿ����������жϣ�
�ʴ�Ϊ����1������©��������̨���Թܣ�
��2������ʱ���Ʒ�Ӧ�ķ�����ֹͣ�����ü��Ӽ�ס�������е���Ƥ�ܣ�����A�м���ˮ���γ�һ��ˮ�������ã����۲쵽һ��ʱ��ˮ��Һ�治�½���˵��װ�����������ã�
��3��2H2O2
2H2O+O2����
��4��AC��
��5���ƾ��ƣ�2KMnO4
K2MnO4+MnO2+O2����
��6�������ſ�������CaCO3+2HCl�TCaCl2+H2O+CO2������ȼ�ŵ�ľ�����ڼ���ƿ�ڣ���ľ��Ϩ��˵���ռ����ˣ�
��7����װ��CO2�ļ���ƿ�����ڷ���ˮ��ˮ���У��۲쵽����ƿ��ˮ��������
��2����װ�ÿ��ڵ����ܵ���Ƥ���ϼ��ϼ��ӣ�ͨ�����ӵĿ�����ʱ���Ʒ�Ӧ�ķ�����ֹͣ������װ�õ������Կ����ü��Ӽ�ס�������е���Ƥ�ܣ�����A�м���ˮ���γ�һ��ˮ�������ã����۲쵽һ��ʱ��ˮ��Һ�治�½���˵��װ�����������ã�
��3������������Һ�ڶ������̵Ĵ�����֮�²�����ˮ���������÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O2
| ||
��4��������ˮ���ռ�����ʱ�������ܿ�һ�����ݾ������ռ������ܻ�����Թ��ڵĿ�����ʹ�ռ�������������װ��©���ᵼ���ռ����������ᵼ����������������ƿ��ûװ��ˮ���ռ����������������ռ�������������
��5���ø��������ȡ��������ȣ��ʻ������Ӿƾ��ƣ���Ӧ�ķ���ʽ�ǣ�2KMnO4
| ||
��6��������̼������ˮ����������ˮ���ռ������ܶȱȿ������������ſ������ռ���ʵ������ȡ������̼�ô���ʯ��ϡ���ᣬ��Ӧ����ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2��������������̼�ɽ�ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��۲�ľ���Ƿ�Ϩ������жϣ�
��7��Ҫ��֤������̼����ˮ���ɽ�װ��CO2�ļ���ƿ�����ڷ���ˮ��ˮ���У��۲켯��ƿ��ˮ���Ƿ����������жϣ�
�ʴ�Ϊ����1������©��������̨���Թܣ�
��2������ʱ���Ʒ�Ӧ�ķ�����ֹͣ�����ü��Ӽ�ס�������е���Ƥ�ܣ�����A�м���ˮ���γ�һ��ˮ�������ã����۲쵽һ��ʱ��ˮ��Һ�治�½���˵��װ�����������ã�
��3��2H2O2
| ||
��4��AC��
��5���ƾ��ƣ�2KMnO4
| ||
��6�������ſ�������CaCO3+2HCl�TCaCl2+H2O+CO2������ȼ�ŵ�ľ�����ڼ���ƿ�ڣ���ľ��Ϩ��˵���ռ����ˣ�
��7����װ��CO2�ļ���ƿ�����ڷ���ˮ��ˮ���У��۲쵽����ƿ��ˮ��������

��ϰ��ϵ�д�
�����Ŀ