��Ŀ����
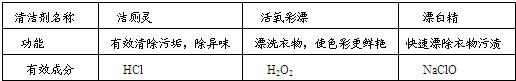
ˮ��һ�ֺܺõ��ܼ������м��ּ�������������ˮ���ܼ����ƶ��ɵģ��书�ܼ���Ч�ɷ����±���ʾ��
| �������� | ����� | ������Ư | Ư�� |
| ���� | ��Ч����۹�������ζ | Ưϴ���ʹɫ�ʸ����� | ����Ư���������� |
| ��Ч�ɷ� | HCl | H2O2 | NaClO |
(1)NaClO����Ԫ�صĻ��ϼ�Ϊ ��
(2)��ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������� ��
(3)������顱�롰Ư�������ܻ��á�����������ײ����ж�������(Cl2)��ͬʱ���Ȼ��ƺ�ˮ���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
(1) +1 �� (2) �����ݲ��� ��
(3) 2HCl + NaClO ="=" Cl2��+ NaCl + H2O ��
���������������1��NaClO�У���Ԫ�صĻ��ϼ���+1�ۣ���Ԫ�صĻ��ϼ���-2�ۣ����������Ԫ�صĻ��ϼ�Ϊ+1�ۣ����+1��
��2�����������ڶ������̵Ĵ���������������������������ݲ�����
��3��������顱�롰Ư��������ܷ�����ѧ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2HCl+NaClO=NaCl+H2O+Cl2����
���㣺���⿼������й�Ԫ�ػ��ϼ۵ļ��㣬ʵ������ȡ�����ķ�Ӧԭ������д��ѧ����ʽ��
�����������������еij�������Ϊ����㣬����������֮��ķ�Ӧ���Լ���ѧ����ʽ��������⣬����ѧ����ѧϰ��ѧ����Ȥ��

��ϰ��ϵ�д�
�����Ŀ