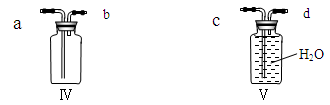��Ŀ����
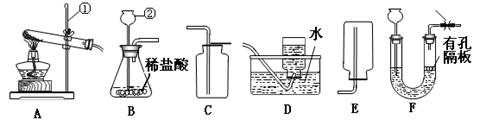
��9�֣�Сǿͬѧѡ������װ�ý���������ʵ�飬����ݲ�����գ�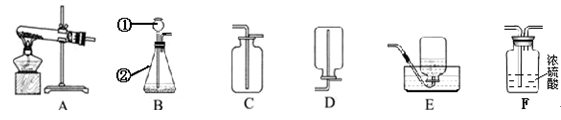
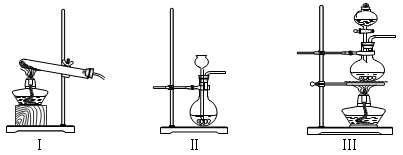
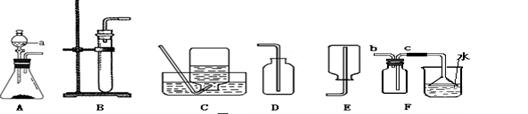
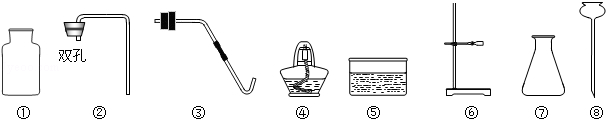
��1��д��ͼ����Ŷ�Ӧ���������ƣ��� ���� ��
��2���ø�����ع�����ȡ�����O2����ѡ�õ�װ������˳���� ��
��Ӧ�Ļ�ѧ����ʽ
��3��Сǿͬѧѡ�ù̡�Һ����ҩƷ������װ��BҲ������ȡ��������Ӧ�Ļ�ѧ����ʽΪ
��4��Сǿͬѧ����B��C��ȡ����һ�ֳ��������壬����Ϊ��ȡ�������� ��
��Ӧ�Ļ�ѧ����ʽ
��5�����Bװ�������ԵIJ����������ǣ�������Ƥ�����Ӻõ����ܣ�Ȼ����Ӽ�ס��Ƥ�ܣ�������м���ˮ���γ�һ��ˮ�������ã����۲쵽___ ��˵�����������á�
��1������©������ƿ��ÿ��1�֣� (���ִ����ֲ�����)��
��2��A F C��2�֣�ȫ�ԲŸ��� �� 2 KMnO4��K2MnO4+ MnO2+ O2����1�֣���
��3�� 2H2O2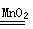 2H2O+ O2�� ��1�֣� (©д�������̲�����) ��
2H2O+ O2�� ��1�֣� (©д�������̲�����) ��
��4��CO2��1�֣� �� ��1�֣�CaCO3 + 2HCl="==" CaCl2 + H2O+ CO2����1�֣���
��5��ˮ���ĸ߶Ȳ���һ��ʱ���ڱ��ֲ��䣨1�֣�(��˼��ͬ����).
����

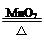 2KCl��3O2��
2KCl��3O2�� MnCl2��Cl2����2H2O
MnCl2��Cl2����2H2O