��Ŀ����
��6�֣�����ͼ��ʾ��ij��ȤѧϰС���ͬѧ���ݡ��������ڵ����ʼ���ɷ�����ѧ��Ӧ������������������ʼ䷴Ӧ�����������������Ϸ�������桰�Ƿ��ӡ�����Ϸ��ʽ��ѧϰ������Ҫ����֮����й�֪ʶ��
(1)�����������Ϸ�����Cu��CO2��CuSO4��Һ��Na2 CO3����Һ����������ѡ���ʺ�ͼ�Тۡ��ܡ�������λ�õ����ʣ��� ���� ���� ��
(2)��ʢ�����ʢݵ���ƿ���ȵ��뼸�η�̪��Һ���ٵ������ʢڲ�������Һ�պ��ʳ���ɫʱ����Һ��pH____7��
(3)���ʢۺ����ʢݷ�����Ӧ�Ļ�ѧ����ʽ��____
��6�֣�(1)��CUS04��Һ ��Na2 C03��Һ��C02 (2)���ڣ���=��
(3)2NaOH +CuS04="==Cu(" OH)2��+Na2S04
����

��ϰ��ϵ�д�
 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�
�����Ŀ
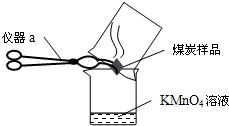 ��
�� 26����֪������Һ��ʹ��ɫ��̪��Һ��죬����ˮ��Һ���м��ԣ�ij��ȤС���������ʵ�飬̽�������˶����й��������ش�ʵ���е��й����⣺
26����֪������Һ��ʹ��ɫ��̪��Һ��죬����ˮ��Һ���м��ԣ�ij��ȤС���������ʵ�飬̽�������˶����й��������ش�ʵ���е��й����⣺ ��2012?�γ�ģ�⣩С��ͬѧ����������ʱ��С�Ľ�һƿ�״������ڻ�¯�Ե�һ�Ѳ�ľ���ϣ������д������������ɣ���������Ȥ��������������ѧϰС���ͬѧ���Բ�ľ�ҵijɷֽ���̽����
��2012?�γ�ģ�⣩С��ͬѧ����������ʱ��С�Ľ�һƿ�״������ڻ�¯�Ե�һ�Ѳ�ľ���ϣ������д������������ɣ���������Ȥ��������������ѧϰС���ͬѧ���Բ�ľ�ҵijɷֽ���̽����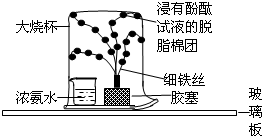 ��֪������Һ��ʹ��ɫ��̪��Һ��죬����ˮ��Һ���м��ԣ�ij��ȤС���������ʵ�飬̽�������˶����й��������ش�ʵ���е��й����⣺
��֪������Һ��ʹ��ɫ��̪��Һ��죬����ˮ��Һ���м��ԣ�ij��ȤС���������ʵ�飬̽�������˶����й��������ش�ʵ���е��й����⣺