��Ŀ����
 θ���������ÿ���ҩ��������ᷴӦ������ͼΪij����ҩ˵���鲿�����ݣ���ȤС��Ը�ҩ�↑չ����̽�����ش��������⣺
θ���������ÿ���ҩ��������ᷴӦ������ͼΪij����ҩ˵���鲿�����ݣ���ȤС��Ը�ҩ�↑չ����̽�����ش��������⣺
I����ҩ������õ�̽����
��֪����̼��þ[��ѧʽΪAlMg��OH��3CO3]�����ᷴӦ�Ļ�ѧ����ʽΪ��
AlMg��OH��3CO3+5HCl�TMgCl2+AlCl3+4H2O+CO2��
��1������Ӧ����������ͨ�����ʯ��ˮ�����۲쵽��������______����Ӧ�Ļ�ѧ����ʽΪ______��
��2���������к���Al3+��Mg2+��OH-��CO32-����ϸ����������ᷴӦ�Ļ�ѧ����ʽ��������ҩ���������ã�����θҺ���ԣ�������______��______���������ţ���
II�����÷�����̽��
�ÿ���ҩΪʲôҪ�������á���С���������ǡ���ͬ������ҩƬ�������ã������ĸ����θ�ᡱ��С������Ϊ�������ҩ�ォ��θҺ�е����ᷴӦ�족��
��3����Ȼ��С������Ǵ���ģ�ԭ����______��
��4��С�����������ʵ����֤���Լ��IJ��룺
[ʵ��1]��һƬ������ҩƬ�����Թ��У������м���5mL 5%��ϡ���
[ʵ��2]��һƬ������ҩƬ���������Թ��У������м���5mL 5%��ϡ���ᣮ
ʵ��ʱ��Ӧ�۲쵽������Ϊ______��
III����Ҫ�ɷֺ�����̽��
��5����ȤС��ȡ3ƬҩƬ���������Թ��У��������������ϡ���ᣬ��ַ�Ӧ��ų�����0.44g����ͨ�����㣬ÿƬҩ���к���AlMg��OH��3CO3�������Ƕ��٣������������0.1��
����֪��ҩ�����������ɷֲ���ϡ���ᷴӦ��AlMg��OH��3CO3����Է�������Ϊ162��
�⣺��1��������̼��ʹ����ʯ��ˮ���������̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+CO2=CaCO3��+H2O��
��2�������������Ӻ����е������ӷ�Ӧ����ˮ��̼������Ӻ����е������ӷ�Ӧ����ˮ�Ͷ�����̼���������Ӻ�þ���Ӳ������е������ӷ�Ӧ����ҩ���������ã�����θҺ���ԣ�������OH-��CO32-��
��3�������ҩƬ����û�����ӣ����Խ������ã��������ĸ����θ�
��4��ʵ��2��ʵ��1��Ӧ�������֤��С���IJ�����ȷ��
��5���⣺6Ƭҩ���к�AlMg��OH��3CO3������Ϊx
AlMg��OH��3CO3+5HCl�TMgCl2+AlCl3+4H2O+CO2��
162 44
x 0.88g
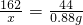
��ã�x=3.24 g
ÿƬҩ���к�AlMg��OH��3CO3������=3.24 g��6=0.5g
��ÿƬҩ���к�AlMg��OH��3CO3������Ϊ0.5 g��
�ʴ�Ϊ����1������ʯ��ˮ����ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
��2��OH-�ͣ�CO32-��
��3�������ҩƬ����û�����ӣ�
��4��ʵ��2��ʵ��1��Ӧ���죻
��5��0.5 g��
��������1�����ݶ�����̼��ʹ����ʯ��ˮ����ǽ��н��
��2�����������������Ӻ�̼������Ӻ����е������ӷ�Ӧ���н��
��3�����ݽ����ҩƬ����û�����ӽ��н��
��4������ʵ��2��ʵ��1��Ӧ�������֤��С���IJ�����ȷ���н��
��5�����ݶ�����̼���������AlMg��OH��3CO3���������������ÿƬҩ���к���AlMg��OH��3CO3���������н��
��������ѧ��Ӧ��ʵ���Ƿ��ӵ����Ѻ�ԭ�ӵ�������ϣ����������Ũ��Խ����˵��Ӧ��֮��ĽӴ����Խ��Ӧ����Ҳ��Խ�죮���Է�Ӧ���ʵĸı��Ҫ�ӽӴ�����ĽǶ�ȥ˼����
��2�������������Ӻ����е������ӷ�Ӧ����ˮ��̼������Ӻ����е������ӷ�Ӧ����ˮ�Ͷ�����̼���������Ӻ�þ���Ӳ������е������ӷ�Ӧ����ҩ���������ã�����θҺ���ԣ�������OH-��CO32-��
��3�������ҩƬ����û�����ӣ����Խ������ã��������ĸ����θ�
��4��ʵ��2��ʵ��1��Ӧ�������֤��С���IJ�����ȷ��
��5���⣺6Ƭҩ���к�AlMg��OH��3CO3������Ϊx
AlMg��OH��3CO3+5HCl�TMgCl2+AlCl3+4H2O+CO2��
162 44
x 0.88g
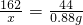
��ã�x=3.24 g
ÿƬҩ���к�AlMg��OH��3CO3������=3.24 g��6=0.5g
��ÿƬҩ���к�AlMg��OH��3CO3������Ϊ0.5 g��
�ʴ�Ϊ����1������ʯ��ˮ����ǣ�Ca��OH��2+CO2=CaCO3��+H2O��
��2��OH-�ͣ�CO32-��
��3�������ҩƬ����û�����ӣ�
��4��ʵ��2��ʵ��1��Ӧ���죻
��5��0.5 g��
��������1�����ݶ�����̼��ʹ����ʯ��ˮ����ǽ��н��
��2�����������������Ӻ�̼������Ӻ����е������ӷ�Ӧ���н��
��3�����ݽ����ҩƬ����û�����ӽ��н��
��4������ʵ��2��ʵ��1��Ӧ�������֤��С���IJ�����ȷ���н��
��5�����ݶ�����̼���������AlMg��OH��3CO3���������������ÿƬҩ���к���AlMg��OH��3CO3���������н��
��������ѧ��Ӧ��ʵ���Ƿ��ӵ����Ѻ�ԭ�ӵ�������ϣ����������Ũ��Խ����˵��Ӧ��֮��ĽӴ����Խ��Ӧ����Ҳ��Խ�죮���Է�Ӧ���ʵĸı��Ҫ�ӽӴ�����ĽǶ�ȥ˼����

��ϰ��ϵ�д�
 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
 θ���������ÿ���ҩ��������ᷴӦ������ͼΪij����ҩ˵���鲿�����ݣ���ȤС��Ը�ҩ�↑չ����̽�����ش��������⣺
θ���������ÿ���ҩ��������ᷴӦ������ͼΪij����ҩ˵���鲿�����ݣ���ȤС��Ը�ҩ�↑չ����̽�����ش��������⣺ ��2012?���ң�θ���������ÿ���ҩ��������ᷴӦ������ͼΪ��̼��þ˵����IJ������ݣ���ȤС��Ը�ҩ�↑չ����̽����
��2012?���ң�θ���������ÿ���ҩ��������ᷴӦ������ͼΪ��̼��þ˵����IJ������ݣ���ȤС��Ը�ҩ�↑չ����̽���� θ���������ÿ���ҩ��������ᷴӦ������ͼΪ��̼��þ˵����IJ������ݣ���ȤС��Ը�ҩ�↑չ����̽����
θ���������ÿ���ҩ��������ᷴӦ������ͼΪ��̼��þ˵����IJ������ݣ���ȤС��Ը�ҩ�↑չ����̽����