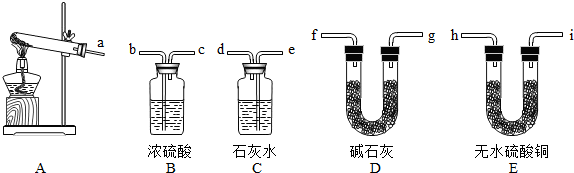
��Ŀ����
��2010?���գ��������ͼ�ش����⣺
��1��ͼ�б��Т٢ڵ��������ƣ���
��2����ʯ��ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽ��
��3����װ��A��װ��C�������һ��װ�ã���װ�ÿ���ȡ��һ��������
��4����ͬ�����£�������NH3�����ܶȱȿ���С����������ˮ�����ȣ��Ȼ�狀��������ƹ����������ȡ����������ȡ����Ӧѡ���װ����

��1��ͼ�б��Т٢ڵ��������ƣ���
����©��
����©��
��������ƿ
����ƿ
����2����ʯ��ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽ��
CaCO3+2HCl=CaCl2+CO2��+H2O
CaCO3+2HCl=CaCl2+CO2��+H2O
����ѡ��ͼ��A
A
��D
D
������ţ���װһ����ȡ������̼��װ�ã����������̼�����ķ�������һ��ȼ�յ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤������
��һ��ȼ�յ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤������
����3����װ��A��װ��C�������һ��װ�ã���װ�ÿ���ȡ��һ��������
������������
������������
���÷�Ӧ�Ļ�ѧ����ʽΪ2H2O2
2H2O+O2������Zn+H2SO4=ZnSO4+H2����
| ||
2H2O2
2H2O+O2������Zn+H2SO4=ZnSO4+H2����
��
| ||
��4����ͬ�����£�������NH3�����ܶȱȿ���С����������ˮ�����ȣ��Ȼ�狀��������ƹ����������ȡ����������ȡ����Ӧѡ���װ����
B
B
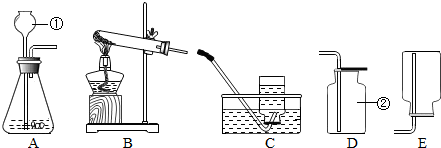
������ţ���������������Ҫ���������̼����������ȡװ�á��ռ�װ�õ�ѡ���Լ�������̼������������ʵ��������ʯ��ʯ��ϡ�����ڳ����£���ȡ������̼�ģ���װ�û���������������������������ֻ̼��һ���ռ������������ſ�������������̼��������������һ��ȼ�յ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤�����ˣ���д��ѧ����ʽʱ��Ҫע����ƽ��
����⣺��1������©���ͼ���ƿ�DZȽϳ��õ������������ʴ�Ϊ������©��������ƿ��
��2��ʵ��������ʯ��ʯ��ϡ�����ڳ����£���ȡ������̼�ģ�����Ҫ���ȣ���ѡA��������̼������ˮ���ܶȱȿ����ܶȴ�ֻ��һ���ռ������������ſ��������ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O��A��D
��3��װ��A��װ��C�������һ��װ�ã���װ�ÿ���ȡ�����������������Ҳ���������������������ڶ��������������������£�����ˮ���������ʴ�Ϊ����������������2H2O2
2H2O+O2������Zn+H2SO4=ZnSO4+H2������
��4���ڼ��ȵ������£��������Ȼ�狀��������ƹ���������ȡ�������ʴ�Ϊ��B
��2��ʵ��������ʯ��ʯ��ϡ�����ڳ����£���ȡ������̼�ģ�����Ҫ���ȣ���ѡA��������̼������ˮ���ܶȱȿ����ܶȴ�ֻ��һ���ռ������������ſ��������ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O��A��D
��3��װ��A��װ��C�������һ��װ�ã���װ�ÿ���ȡ�����������������Ҳ���������������������ڶ��������������������£�����ˮ���������ʴ�Ϊ����������������2H2O2
| ||
��4���ڼ��ȵ������£��������Ȼ�狀��������ƹ���������ȡ�������ʴ�Ϊ��B
��������������Ҫ���������̼���Ʒ����������ռ������������ʵ�鷽��̽��������������ռ�������������Ҫ���������Ŀ������������Ϣ��ѡ����ʵ�װ�ò��������ӣ���������������ȡ�ͼ���Ҳ���п�����Ҫ����֮һ��ͬѧ��Ӧ���������գ�����Ӧ�ã���������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ