��Ŀ����
��2010?���գ���ʽ̼��ͭ[Cu2��OH��2CO3]���׳ƿ�ȸʯ��ͭ�̣���ͭ������е�������������̼��ˮ��Ӧ���������ʣ���ʽ̼��ͭ���ȷ����ֽ⣬�����������������ʦΪ����ͬѧ��̽����ʽ̼��ͭ������ȫ�ֽ��IJ����ͬѧ����������������ҩƷ����Ƥ�����ɣ����ԣ�������֪��ʯ�ҵ���Ҫ�ɷ��������ƺ��������ƣ����������ʵ�飺
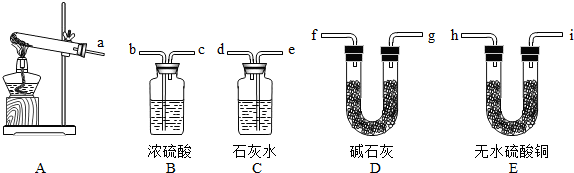
��1��ʵ��ǰ������A�з�Ӧ���������ԵIJ�����
��2��Ϊ����֤�����е����壬�罫����������ͨ��װ��
��3����һ���������������֤������ʹʵ�����ѧ������Ӧ�ڣ�2������ѡ������װ���м�����
��4����֤�����еĺ�ɫ���壬Ӧѡ��
��5����ʽ̼��ͭ���ȷֽ�Ļ�ѧ����ʽ

��1��ʵ��ǰ������A�з�Ӧ���������ԵIJ�����
������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã�
������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã�
����2��Ϊ����֤�����е����壬�罫����������ͨ��װ��
C
C
����װ����ţ���ͬ����������ʯ��ˮ�����
ʯ��ˮ�����
����֤���������ж�����̼��������е�����ʹE
E
װ���е����ʱ���ɫ����֤����������ˮ
ˮ
����3����һ���������������֤������ʹʵ�����ѧ������Ӧ�ڣ�2������ѡ������װ���м�����
B
B
װ�ã�Ŀ�������������е�ˮ
���������е�ˮ
����4����֤�����еĺ�ɫ���壬Ӧѡ��
A
A
װ�ý���ʵ�飬����Ҫһ�ֹ��巴Ӧ����ľ̿��
ľ̿��
��д���ƣ�����5����ʽ̼��ͭ���ȷֽ�Ļ�ѧ����ʽ
Cu2��OH��2CO3
2CuO+CO2��+H2O
| ||
Cu2��OH��2CO3
2CuO+CO2��+H2O
-���÷�Ӧ��������
| ||
�ֽⷴӦ
�ֽⷴӦ
����������1�����������Լ��ԭ�������γ�ѹǿ�����������������
��2�����������̼�ó���ʯ��ˮ������ˮ����ˮ����ͭ��
��3������ÿ�μ���һ�������˼·���
��4����������ͭ����̼������ԭ��Ӧ���
��5�����ݷ�Ӧ��������P��д��ѧ����ʽ��ԭ���𣬷ֽⷴӦ��һ��࣮
��2�����������̼�ó���ʯ��ˮ������ˮ����ˮ����ͭ��
��3������ÿ�μ���һ�������˼·���
��4����������ͭ����̼������ԭ��Ӧ���
��5�����ݷ�Ӧ��������P��д��ѧ����ʽ��ԭ���𣬷ֽⷴӦ��һ��࣮
����⣺��1����װ�������Լ�鷽��Ϊ������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã�
��2�����������̼�ó���ʯ��ˮ��������̼ʹ����ʯ��ˮ����ǣ���ˮ����ͭ��ˮ��������
��3����ͨ��Eװ��֤��ˮ�Ĵ��ڣ�Ȼ��ͨ��Bװ�ó�ȥʣ���ˮ���ٽ�ʣ������ͨ��C����ʯ��ˮ��֤������̼��
��4����ɫ��������ͭ����ľ̿�۷�Ӧ���ɺ�ɫ��ͭ����ʹ����ʯ��ˮ����ǵ����������̼�����������Ӧѡ��Aװ�ã�
��5����ʽ̼��ͭ�ֽ���������ͭ��ˮ�Ͷ�����̼����ѧ����ʽΪ��Cu2��OH��2CO3
2CuO+CO2��+H2O����Ӧ����һ�֣������������֣�һ��࣬���ڷֽⷴӦ��
�ʴ�Ϊ����1��������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã���2��C��ʯ��ˮ����ǣ�E��ˮ
��3��B ���������е�ˮ
��4��A��ľ̿��
��5��Cu2��OH��2CO3
2CuO+CO2��+H2O���ֽⷴӦ
��2�����������̼�ó���ʯ��ˮ��������̼ʹ����ʯ��ˮ����ǣ���ˮ����ͭ��ˮ��������
��3����ͨ��Eװ��֤��ˮ�Ĵ��ڣ�Ȼ��ͨ��Bװ�ó�ȥʣ���ˮ���ٽ�ʣ������ͨ��C����ʯ��ˮ��֤������̼��
��4����ɫ��������ͭ����ľ̿�۷�Ӧ���ɺ�ɫ��ͭ����ʹ����ʯ��ˮ����ǵ����������̼�����������Ӧѡ��Aװ�ã�
��5����ʽ̼��ͭ�ֽ���������ͭ��ˮ�Ͷ�����̼����ѧ����ʽΪ��Cu2��OH��2CO3
| ||
�ʴ�Ϊ����1��������û��ˮ�У����ֽ����Թ���ڣ��۲쵼�ܿڲ������ݣ���ȴ����������һ��ˮ����˵�����������ã���2��C��ʯ��ˮ����ǣ�E��ˮ
��3��B ���������е�ˮ
��4��A��ľ̿��
��5��Cu2��OH��2CO3
| ||
������Ҫͬʱ���������̼��ˮ����Ҫ�ȼ���ˮ�ټ��������̼������ˮ����ˮ����ͭ��

��ϰ��ϵ�д�
�����Ŀ