��Ŀ����
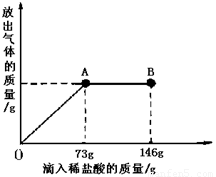
(9��)��֪ Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��21.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10����ϡ���ᡣ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺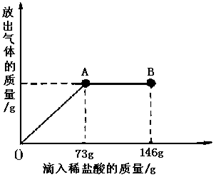
(1)���μ�ϡ������ͼ��A��ʱ���ų������������ g
(2)���μ�ϡ������ͼ��B��ʱ���ձ�����Һ�������� ��
(3)���μ�ϡ������ͼ��A��ʱ���ձ���Ϊ��������Һ(����)��ͨ����������������ʵ�����������
��1��4.4 ��2��NaCl HCl ��3��25%
����

��ϰ��ϵ�д�
�����Ŀ