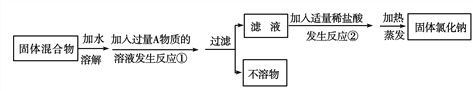
��Ŀ����
����Ŀ��2017��5��18�գ��ҹ��״κ���ȼ�����Բɳɹ�������ȼ��������Ҫ�ɷ��Ǽ��顣
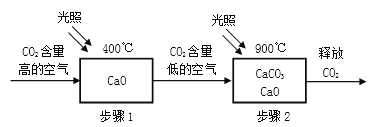
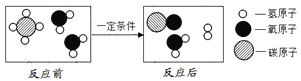
��1���ý��ʯ���ɵ���ͷ���ɺ���ȼ�����������˽��ʯ��____________�����������ʣ���
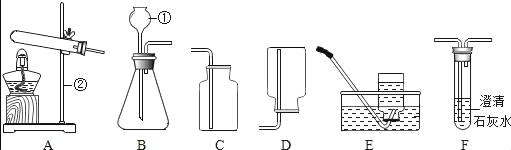
��2��������ʵ������ȡ����ʱ����ͼװ���ռ���������Ӧ�ô�_________ͨ�루�m����n������

��3����ͼΪ������ˮ��Ӧ����ʾ��ͼ����д����Ӧ�Ļ�ѧ����ʽ_________________��
��4������ȼ�շ���ȷ�������Ԫ����ɡ�
����д��������ȫȼ�յĻ�ѧ����ʽ____________________��
����д��ȷ������Ԫ����ɵ�ʵ�鷽��______________����Ҫд��ʵ������������ۣ���
��5����ú�������ɽ���ú�����ģ����ٿ�����Ⱦ����Ҳ����˽���ġ����ġ�����ȼ���Ŀ��ɽ��Ỻ����һ״������֪��1kg������ȫȼ�շų�����55700kJ��1kg����5%�ı�ú��ȫȼ�շų�����29000kJ������㣺
��1kg������ȫȼ�շų���������___________ǧ�˺���5%�ı�ú��ȫȼ�շų�������ȣ�����С�������λ����
����ȫȼ��1kg���飬�����Ͽɼ����ŷŶ�������________ǧ�ˣ�
���𰸡� Ӳ�ȴ� n CH4��H2O ![]() CO��3H2 CH4 + 2O2
CO��3H2 CH4 + 2O2 ![]() CO2 + 2H2O �鴿��ȼ���飬�øɶ���ĵ�С�ձ���ס���棬�ձ�����ˮ����˵��������Ԫ�أ�Ѹ�ٵ�ת�ձ���������������ʯ��ˮ������ʯ��ˮ����ǣ�˵������̼Ԫ�ء��������������𰸣� 1.92 k g 0.19 kg
CO2 + 2H2O �鴿��ȼ���飬�øɶ���ĵ�С�ձ���ס���棬�ձ�����ˮ����˵��������Ԫ�أ�Ѹ�ٵ�ת�ձ���������������ʯ��ˮ������ʯ��ˮ����ǣ�˵������̼Ԫ�ء��������������𰸣� 1.92 k g 0.19 kg
����������1�����ʯ����Ȼ����Ӳ�����ʣ��ý��ʯ���ɵ���ͷ���ɺ�������ȼ�����������˽��ʯ��Ӳ�ȴ����������2��������ܶȱȿ���С��Ӧ�ӳ��ܽ��룬������Ӧ�ô�nͨ������3���ɼ�����ˮ��Ӧ����ʾ��ͼ��֪��������ˮ��Ӧ����һ����̼����������Ӧ�Ļ�ѧ����ʽΪ��CH4��H2O![]() CO��3H2����4���ټ���ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4 + 2O2
CO��3H2����4���ټ���ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4 + 2O2 ![]() CO2 + 2H2O���ڼ����ǿ�ȼ�����壬��ȼǰҪ���鴿�ȣ�ȷ������Ԫ����ɵ�ʵ�鷽��Ϊ���鴿��ȼ���飬�øɶ���ĵ�С�ձ���ס���棬�ձ�����ˮ����˵��������Ԫ�أ�Ѹ�ٵ�ת�ձ���������������ʯ��ˮ������ʯ��ˮ����ǣ�˵������̼Ԫ������5������1kg������ȫȼ�շų���������
CO2 + 2H2O���ڼ����ǿ�ȼ�����壬��ȼǰҪ���鴿�ȣ�ȷ������Ԫ����ɵ�ʵ�鷽��Ϊ���鴿��ȼ���飬�øɶ���ĵ�С�ձ���ס���棬�ձ�����ˮ����˵��������Ԫ�أ�Ѹ�ٵ�ת�ձ���������������ʯ��ˮ������ʯ��ˮ����ǣ�˵������̼Ԫ������5������1kg������ȫȼ�շų���������![]() ǧ�˺���5%�ı�ú��ȫȼ�շų����������
ǧ�˺���5%�ı�ú��ȫȼ�շų���������� ![]() ��29000kJ=55700kJ��
��29000kJ=55700kJ�� ![]() =1.92 kg��������ȫȼ��1kg���飬�����Ͽɼ����ŷŶ�������
=1.92 kg��������ȫȼ��1kg���飬�����Ͽɼ����ŷŶ�������![]() ǧ����S+O2
ǧ����S+O2 ![]() SO2
SO2
32 64
1.92��5% ![]()
![]()
![]() =0.19kg��
=0.19kg��
����1kg������ȫȼ�շų���������1.92ǧ�˺���5%�ı�ú��ȫȼ�շų��������
����ȫȼ��1kg���飬�����Ͽɼ����ŷŶ�������0.19ǧ����

����Ŀ���Ķ�������ն��ġ�
��̸ˮ���ı���
�������֦���ٿţ����dz��������ˡ���������ʫ��������֦������ϲ����֦֮�顣ˮ��������������ζ���ϵ��������ܣ������ṩ�ḻ��Ӫ����������ʱˮ���ı���Ҳ������Ǵ���СС�ķ��գ����治����ˮ����ʧˮ���ñ��ʡ�
��ˮ���ı����¶��´��أ��ܽϳ�ʱ�䱣���ʹ����е�Ʒ�ʺ�Ӫ����������б��´��ء�Ϊ��̽����֦�ı��´��أ���֦�ı����¶�Ϊ-1.2�棩�Ƿ�������ͨ��أ��¶�ͨ��Ϊ0~10�棩��������Ա�����һ��ʵ�飬ʵ���������1��ʾ������֦�Ļ�ԭ�Ǻ�����Ϊ������֦Ʒ�ʱ仯��ָ�꣨��ԭ�Ǻ���Խ�ߣ�Ʒ��Խ�ã���ÿ���������һ��ˮ��Ʒ�ʼ�⣬ʵ������ͼ1��
��1 ʵ������
��� | �����¶� | ʪ�� | ��ע |
1 | -1.2�� | 87% | ��֦��Ʒ�֡���С������ȡ������Լ�������������ͬ������ |
2 | 3�� | 87% |

ͼ1 ��ԭ�Ǻ����仯
������Ũ��ҲӰ����ˮ���ı��ʡ��ڴ���ˮ��ʱΪ�����ƺ������ã�һ��Ҫ����������Ũ�ȣ���������̼�ͷ�����Сʱ������������������ʱ��Ӧ������Ũ������ˮ���Ĵ��档�������̫�ͣ�ֲ����֯�ͽ����������������������IJ���������ϸ����һ���Ķ������ã�Ӱ���߲�ˮ���ı��ʡ�
���⣬����ʱ��Ҫע����Щˮ�����ܺ������߹�һ���ţ���ƻ����ľ�ϡ��㽶�ȡ�����ˮ���ڳ�������л��ͷš���ϩ�����壬�ɼ���ˮ���ij�����ϻ���������ˮ��Ҳ���ͷ���ϩ�������һ��ˮ���У������һ���ǻ��ģ�Ҫ��������ȥ��
�����������ݣ��ش��������⣺
��1����ϩ___________����ܡ����ܡ�������ˮ����
��2�������е���֦ʵ���У��о���Ӱ��ˮ�����ص������� ________��
��3��ͨ��ͼ1�ɵõ�����Ϣ��________��д��1�����ɣ���
��4������˵������ȷ����________��
A����֦�Ļ�ԭ�Ǻ����ڱ��´���ʱʼ�ո�����ͨ����
B������Ũ��Խ��Խ������ˮ���ı���
C����ͨ����¶�ָ����3��
��5����ٳ��ճ�������ˮ�����ʵķ���_________��д��һ�ּ��ɣ���