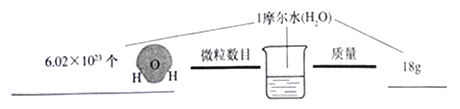
��Ŀ����
����Ŀ����ѧ���츣������Ŀ�ѧ����������ѧ֪ʶ�ش��������⣺
��ѧ������ϢϢ���
��1���ɻ��͵�ʳƷ�����������⡢������ţ�̵ȣ��������и�����Ӫ������_____�����е�Ӫ���������������ϵͳ�о�ø�Ĵ����ã���������Ϊ_____���ѧʽ����
��2�����һЩ���岻���ʵij˿ͣ�����������䱸��ð����ҩ��θҩ���������ȳ���ҩ�θҩ����һ��������þƬ��������ҩ�����к�θ������θ�ᣬ��ԭ����_____���û�ѧ����ʽ��ʾ����
��ѧָ���������������Դ
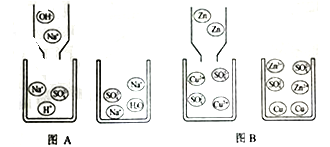
������̼�ġ������롰��桱��ʵ������������ŵ���Ҫ;��֮һ��ʵ�������У�������������NaOH��Һ��������CO2������ͼ��ͼ��ʾ����������������δ�������
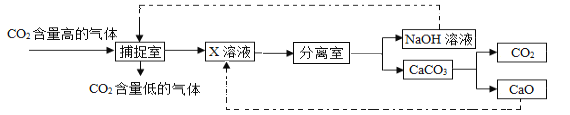
��3�������з�Ӧ����ʽ��_____��
��4��������ͼ��δ�漰�Ļ�����Ӧ������_____��Ӧ�����������������У���ѭ��ʹ�õ�������_____��
��ѧ�ٽ���ѧ�����ķ�չ
��5����Eu��������ɫ���ӻ���ӫ��ۣ��Ǽ��⼰ԭ����Ӧ�õ���Ҫ���ϡ���֪һ�����ʵĽ������ڿ����е�ȼ������ȼ�գ�������ɫ���棬���ȣ��н��ư�ɫ�Ĺ������ɣ���ͼ�����������ϻش�

����ȼ������������Eu2O3���Ļ�ѧ����ʽΪ_____��
����Ľ�����Ա�ͭ_____���ǿ������������
��6��2017��5��9�գ��ҹ��������·��ֵ�����Ԫ�ص��������ơ�����113��Ԫ�ص���������ȷ��Ϊ���b������Ԫ�ط���ΪNh���bԭ�ӵ����ԭ������Ϊ278������������Ϊ3�����bԭ�Ӻ�����_____���ӣ��bԭ���γɵ����ӷ���Ϊ_____��
���𰸡����ࣨ����ۣ� C6H12O6 Mg(OH)2+2HCl=MgCl2+2H2O 2NaOH+CO2=Na2CO3+H2O �û� CaO��NaOH 4Eu+3O2![]() Eu2O3 ǿ 165 Nh3+
Eu2O3 ǿ 165 Nh3+
��������
��ѧ������ϢϢ���
��1�����и�����Ӫ���������ࣨ����ۣ������е�Ӫ���������������ϵͳ�о�ø�Ĵ����ã���������Ϊ�����ǣ���ѧʽΪ��C6H12O6��������ࣨ����ۣ���C6H12O6
��2�����һЩ���岻���ʵij˿ͣ�����������䱸��ð����ҩ��θҩ���������ȳ���ҩ�θҩ����һ��������þƬ��������ҩ�����к�θ������θ�ᣬ������þ�����ᷴӦ�����Ȼ�þ��ˮ���仯ѧ����ʽΪ��Mg(OH)2+2HCl=MgCl2+2H2O�����Mg(OH)2+2HCl=MgCl2+2H2O
��ѧָ���������������Դ
��3�����������������������̼��Ӧ����̼���ƺ�ˮ����Ӧ����ʽ�ǣ�2NaOH+CO2=Na2CO3+H2O�����2NaOH+CO2=Na2CO3+H2O
��4��������Ӧ���������֣�����ͼ���У����Ϸ�Ӧ�����ֽⷴӦ���ֽⷴӦ��δ�漰�Ļ����û���Ӧ�����������������У���ѭ��ʹ�õ��������������ƣ�������CO2��������������X��Һ��Ӧ������û���CaO��NaOH
��ѧ�ٽ���ѧ�����ķ�չ
��5������ȼ������������Eu2O3���Ļ�ѧ����ʽΪ4Eu+3O2![]() Eu2O3�����4Eu+3O2
Eu2O3�����4Eu+3O2![]() Eu2O3
Eu2O3
�����ʵĽ������ڿ����е�ȼ������ȼ�գ���ͭ�����ٿ�����ȼ�գ�˵����Ľ�����Ա�ͭǿ�����ǿ
��6��2017��5��9�գ��ҹ��������·��ֵ�����Ԫ�ص��������ơ�����113��Ԫ�ص���������ȷ��Ϊ���b������Ԫ�ط���ΪNh���bԭ�ӵ����ԭ������Ϊ278������������Ϊ3�����bԭ�Ӻ���������278-113=165�����bԭ������������Ϊ3��������ʧȥ3�����ӣ��γɵ����ӷ���ΪNh3+�����165��Nh3+

 �Ķ��쳵ϵ�д�
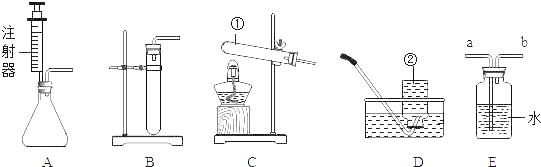
�Ķ��쳵ϵ�д�����Ŀ��ij��ѧ��ȤС���ͬѧѧϰ�������ԭ������,���������ͼ��ʾʵ�飬���Է�Ӧ��ƿ�в�����Һ����̽����
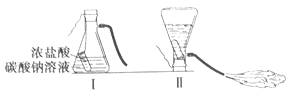
��������⣩��Һ������������ʲô?
����������裩
����1:��Һ�е�������NaCl��Na2CO3��HCl
����2:��Һ�е�����ֻ��NaCl
����3:��Һ�е�������NaCl��HCl
����4:��Һ�е�������______��
�������뽻����
С����Ϊ����I������֤��֪���Ǵ���ģ�����������_____(���û�ѧ����ʽ˵��)��
��ʵ������ۣ�
(1)С��ͬѧΪ��֤����3��ȡ������Һװ���Թ��У�Ȼ������̪��Һ��������Һ����ɫ������С����Ϊ����3��ȷ������Ϊ���Ľ���____(������ȷ������������),������______________��
���������ʵ�鷽����֤����3:
ʵ�鲽�� | ʵ������ | ʵ����� |
__________________ | __________________ | ����3��ȷ |
(2)��֤����4����ѡ����Լ���____________��
A.��̪��ҺB.����������ҺC.ϡ����D.�Ȼ�����Һ
����չ��Ӧ�ã�����Һ�е�������NaCl��HCl��������������ʣ����������Լ���ֻҪ�Է�Һ����____���������ɴӷ�Һ�еõ�NaCl���塣