��Ŀ����
��ͼ��ʾΪʵ�����г����������Ʊ�������ռ���̽���������ʵij����������Ը�����ĿҪ�ش��������⣺

��1�����Թ���������Һ�Ͷ�������Ϊԭ����ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ
����������ʱ����������Ӧ�Ļ�ѧ����ʽΪ
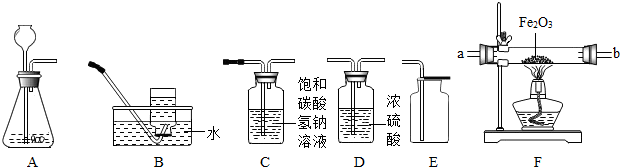
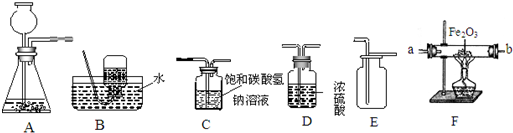
��Aװ���г���©���¶˹ܿڲ���Һ�����µ�ԭ����
��2������װ��B��C��������֤CO�Ļ�ԭ�ԣ�COͨ��װ��Bʱ���Կ�����������
��3����CO2����ͨ��װ��E�У���װ��E����Һ��������֮ǰ��Ȼ�
A����С B�� ���� C������ D�����жϣ�

��1�����Թ���������Һ�Ͷ�������Ϊԭ����ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ
A��D��F
A��D��F
����д���������ĸ��������������ʱ����������Ӧ�Ļ�ѧ����ʽΪ
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��Aװ���г���©���¶˹ܿڲ���Һ�����µ�ԭ����
��ֹ���ɵ�����ӳ���©�����ݳ�
��ֹ���ɵ�����ӳ���©�����ݳ�
����2������װ��B��C��������֤CO�Ļ�ԭ�ԣ�COͨ��װ��Bʱ���Կ�����������
��ɫ����ͭ��Ϊ��ɫ
��ɫ����ͭ��Ϊ��ɫ
��װ��C�г��ְ�ɫ������д��װ��C�з�����Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+CO2=CaCO3��+H2O
Ca��OH��2+CO2=CaCO3��+H2O
����3����CO2����ͨ��װ��E�У���װ��E����Һ��������֮ǰ��Ȼ�
C
C
����д��ţ���A����С B�� ���� C������ D�����жϣ�
��������1������ʵ�����Ʊ�������ʵ�鲽�衢ԭ���Լ�ע��������н��
��2������һ����̼��ԭ��������ʵ�������Լ�������̼��ʯ��ˮ��Ӧ����̼��ƺ�ˮ���н��
��3�����ݶ�����̼�ܺ��������Ʒ�Ӧ���н��
��2������һ����̼��ԭ��������ʵ�������Լ�������̼��ʯ��ˮ��Ӧ����̼��ƺ�ˮ���н��
��3�����ݶ�����̼�ܺ��������Ʒ�Ӧ���н��
����⣺��1�����ɹ���������Һ�Ͷ�������Ϊԭ����ʵ�������Ʊ����ռ����������ʵ�鲽���֪����ѡ����������˳��ΪA��D��F������������Ӧ�Ļ�ѧ����ʽΪ2H2O2
2H2O+O2����
��Aװ���г���©���¶˹ܿڲ���Һ�����µ�ԭ���Ƿ�ֹ���ɵ�����ӳ���©�����ݳ���
��2������װ��B��C��������֤CO�Ļ�ԭ�ԣ�COͨ��װ��Bʱ���Կ����������Ǻ�ɫ����ͭ��Ϊ��ɫ��������̼��ʯ��ˮ��Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+CO2=CaCO3��+H2O��
��3��������̼�ܺ��������Ʒ�Ӧ����̼���ƺ�ˮ���������������ӣ�
�ʴ�Ϊ����1����A��D��F�� ��2H2O2
2H2O+O2�����۷�ֹ���ɵ�����ӳ���©�����ݳ���
��2����ɫ����ͭ��Ϊ��ɫ�� Ca��OH��2+CO2=CaCO3��+H2O��
��3��C��
| ||
��Aװ���г���©���¶˹ܿڲ���Һ�����µ�ԭ���Ƿ�ֹ���ɵ�����ӳ���©�����ݳ���
��2������װ��B��C��������֤CO�Ļ�ԭ�ԣ�COͨ��װ��Bʱ���Կ����������Ǻ�ɫ����ͭ��Ϊ��ɫ��������̼��ʯ��ˮ��Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+CO2=CaCO3��+H2O��
��3��������̼�ܺ��������Ʒ�Ӧ����̼���ƺ�ˮ���������������ӣ�
�ʴ�Ϊ����1����A��D��F�� ��2H2O2
| ||
��2����ɫ����ͭ��Ϊ��ɫ�� Ca��OH��2+CO2=CaCO3��+H2O��
��3��C��
���������ʵ������ȡ������������̼�ķ�Ӧԭ����ʵ�鲽�衢װ��ʾ��ͼ��ע�������Լ����ʵļ���ͳ��ӵ�֪ʶ���������е�֪ʶ���н��Ҫ��ͬѧ����ƽʱ��ѧϰ�м�ǿ����֪ʶ�Ĵ������Ա��ܹ����Ӧ�ã�

��ϰ��ϵ�д�
 ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�
�����Ŀ



 2Fe+3CO2��
2Fe+3CO2��