��Ŀ����
2012��3��22���ǵڶ�ʮ��������ˮ�ա���ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ����ˮ��Դ���˽�ˮ���й�֪ʶ����1��ȡһ�ݻ��ǵ�ˮ���������м������������ܽ⣬����һ��ʱ�����______����������ƣ�����ȥ����С������������Һ�м������̿��������______�Գ�ȥ��ɫ����ζ�ȣ�
��2��Ϊ�ж�����ˮ����Ӳˮ������ˮ��______���飮ˮ��Ӳ�ȹ����Ӱ�����������Ӧ������ʹ�ã������г�����______�ķ�����Ӳˮ��������ʵ��������Ҫ�õ������̶���ߵ�ˮ������______�ķ�����
��3������ˮ��Դ����Լ��ˮ��ȫ�������Σ�Ӧ�ᳫ���·�ʽ�е�______������ĸ��ţ���
A��������ˮ��ϴ��ˮ��������������� B������ϵط�ˮϴ�·�
C������ϵط�ˮˢ�� D���ִ�ũ����ֲ�����ࡢ�ι�ȷ�ʽ
��4����������A��B��C��D���dz��л�ѧ�еij������ʣ�����֮�������ͼ��ʾ��ת����ϵ����Ӧ��������Щ��Ӧ����������ȥ��������A��B�����Ԫ����ͬ��C��ʹ�����ǵ�ľ����ȼ��D��һ����ɫ���嵥�ʣ�
����д���������ʵĻ�ѧʽ��BΪ______��CΪ______��
��д�����з�Ӧ�Ļ�ѧ����ʽ��B��D______ 2H2��+O2��

���𰸡���������1�����ݹ����dz�ȥ�����������Լ�����̿���������Խ��н��
��2�������ж�����ˮ����Ӳˮ������ˮ���÷���ˮ�������г�������еķ�����Ӳˮ�����Լ������̶���ߵ�ˮ����������ķ������н��
��3��ֻҪ���Ͻ�Լ��ˮ���У�
��4������A��B�����ʵ�Ԫ��������ͬ��������֤�����֣�ʵ������˫��ˮ���������ʽ��н��
����⣺��1�������dz�ȥ���������ʣ�����ȡһ�ݻ��ǵ�ˮ���������м������������ܽ⣬����һ��ʱ����й��ˣ�����̿���������ԣ��ܳ�ȥ��ɫ����ζ��
��2���ж�����ˮ����Ӳˮ������ˮ���÷���ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ�������г�������еķ�����Ӳˮ�����������̶���ߵ�ˮ����������ķ�����
��3��A��������ˮ��ϴ��ˮ���������������������һˮ���ã����Խ�Լ��ˮ�� B������ϵط�ˮϴ�·��������˷�ˮ��Դ��C������ϵط�ˮˢ���������˷�ˮ��Դ��D���ִ�ũ����ֲ�����ࡢ�ι�ȷ�ʽ�����Խ�Լ��ˮ��
��4����֪��A��D���dz��л�ѧ�еij������ʣ�A��B��������Ԫ��������ͬ��C��ʹ�����ǵ�ľ����ȼ��C��������������AΪ˫��ˮ��˫��ˮ�ڶ������̴�����������ˮ��������ˮ�������������������������������ȼ������ˮ����A��H2O2��B��H2O��C��O2��D��H2����������۴���ԭ�⣬������֤�����÷��ϣ�
�ʴ�Ϊ��
��1�����ˣ� ������
��2������ˮ����У����� ��3��AD��
��4����H2O��O2����2H2O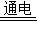 2H2��+O2���� 2H2+O2
2H2��+O2���� 2H2+O2  2H2O��
2H2O��
�����������ؼ���Ҫ֪������ˮ�ľ������̣�֪�������仯�뻯ѧ�仯�������˽���ˮ������֪�������г�����Լ��ˮ�ķ�����
��2�������ж�����ˮ����Ӳˮ������ˮ���÷���ˮ�������г�������еķ�����Ӳˮ�����Լ������̶���ߵ�ˮ����������ķ������н��
��3��ֻҪ���Ͻ�Լ��ˮ���У�
��4������A��B�����ʵ�Ԫ��������ͬ��������֤�����֣�ʵ������˫��ˮ���������ʽ��н��
����⣺��1�������dz�ȥ���������ʣ�����ȡһ�ݻ��ǵ�ˮ���������м������������ܽ⣬����һ��ʱ����й��ˣ�����̿���������ԣ��ܳ�ȥ��ɫ����ζ��
��2���ж�����ˮ����Ӳˮ������ˮ���÷���ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ�������г�������еķ�����Ӳˮ�����������̶���ߵ�ˮ����������ķ�����
��3��A��������ˮ��ϴ��ˮ���������������������һˮ���ã����Խ�Լ��ˮ�� B������ϵط�ˮϴ�·��������˷�ˮ��Դ��C������ϵط�ˮˢ���������˷�ˮ��Դ��D���ִ�ũ����ֲ�����ࡢ�ι�ȷ�ʽ�����Խ�Լ��ˮ��
��4����֪��A��D���dz��л�ѧ�еij������ʣ�A��B��������Ԫ��������ͬ��C��ʹ�����ǵ�ľ����ȼ��C��������������AΪ˫��ˮ��˫��ˮ�ڶ������̴�����������ˮ��������ˮ�������������������������������ȼ������ˮ����A��H2O2��B��H2O��C��O2��D��H2����������۴���ԭ�⣬������֤�����÷��ϣ�
�ʴ�Ϊ��
��1�����ˣ� ������
��2������ˮ����У����� ��3��AD��
��4����H2O��O2����2H2O
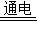 2H2��+O2���� 2H2+O2
2H2��+O2���� 2H2+O2  2H2O��
2H2O�������������ؼ���Ҫ֪������ˮ�ľ������̣�֪�������仯�뻯ѧ�仯�������˽���ˮ������֪�������г�����Լ��ˮ�ķ�����

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ

 2012��3��22���ǵڶ�ʮ�조����ˮ�ա���ˮ����������������������أ�
2012��3��22���ǵڶ�ʮ�조����ˮ�ա���ˮ����������������������أ�