��Ŀ����
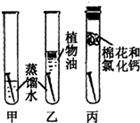
ijʵ��С������ijŨ�ȵ�����ʹ���ʯΪԭ�ϣ�����ͼI��ʾװ���Ʊ������������̼��
��֪��������̼�ڱ���̼��������Һ�е��ܽ�Ⱥ�С

��1��������̼��Ʒ�Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ��ǰ�����ݶ�����̼��ʯ��ˮ�ķ�Ӧ����С��ͬѧԤ����B��Ӧ�۲쵽�������� ��
��3��ʵ�鿪ʼ��B�в�δ�۲쵽�����������ۺ�С��ͬѧ��A��Bװ��֮��������Cװ�ã����¿�ʼʵ�飬��B�й۲쵽��Ԥ���������ϻ�ѧ����ʽ��������˵��Cװ�õ����ã� ��
��4����Cװ����װ������̼��������Һ�����������ռ�������̼���������̼Ӧ�� ����a��b���ڽ���C��
��֪��������̼�ڱ���̼��������Һ�е��ܽ�Ⱥ�С

��1��������̼��Ʒ�Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ��ǰ�����ݶ�����̼��ʯ��ˮ�ķ�Ӧ����С��ͬѧԤ����B��Ӧ�۲쵽�������� ��
��3��ʵ�鿪ʼ��B�в�δ�۲쵽�����������ۺ�С��ͬѧ��A��Bװ��֮��������Cװ�ã����¿�ʼʵ�飬��B�й۲쵽��Ԥ���������ϻ�ѧ����ʽ��������˵��Cװ�õ����ã� ��
��4����Cװ����װ������̼��������Һ�����������ռ�������̼���������̼Ӧ�� ����a��b���ڽ���C��
��1��CaCO3 + 2HCl ="==" CaCl2 + CO2��+ H2O ��2��������ɫ����
��3���������CO2�е�HCl��ȥ��1�֣�NaHCO3 + HCl ="==" Na2CO3 + CO2��+ H2O
��4��b
��3���������CO2�е�HCl��ȥ��1�֣�NaHCO3 + HCl ="==" Na2CO3 + CO2��+ H2O
��4��b
�����������1��������̼��Ʒ�Ӧ�������Ȼ��ơ�ˮ�Ͷ�����̼���ʷ�Ӧ�Ļ�ѧ����ʽΪCaCO3 + 2HCl = CaCl2 + CO2��+ H2O��
��2�����ڶ�����̼����ʯ��ˮ�ķ�Ӧ����̼��Ƴ���������B��Ӧ�۲쵽��������ʯ��ˮ����ǡ�
��3������������лӷ��ԣ��ʷ�Ӧ���ɵĶ�����̼�����л����HCl���壬������HCl�Ķ�����̼�������ʯ��ˮʱ��������̼��ʯ��ˮ��Ӧ���ɵ�̼��Ƴ�������HCl����ˮ��������ᣬ�������ܷ�Ӧ�ٴ������Ȼ��ơ�ˮ�Ͷ�����̼����ʵ�鿪ʼ��B�в�δ�۲쵽����������A��Bװ��֮��������Cװ�ú�����B�й۲쵽��Ԥ������˵��Cװ�ó�ȥ��HCl���壬��NaHCO3 + HCl ="==" Na2CO3 + CO2��+ H2O��
��4������Cװ����װ������̼��������Һ�������ռ�������̼���壬���ڶ�����̼������ܶ�С��Һ����ܶȣ���Ӧ�ӽ϶̵ĵ���bͨ�룬���½���Һ�ӽϳ��ĵ����ų���
��������ѧ����ʵ��Ϊ������ѧ�ƣ���Ƕ�����̼��ʵ�����Ʒ���ԭ���Ͳ���ע������ȣ��ǽ���Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 x Fe + y H2O��Fe3C + 2 H2
x Fe + y H2O��Fe3C + 2 H2 3 Fe + CH4��
3 Fe + CH4��
 3 CO2 + 6H2O + 8 Fe������ÿ����Ӧ����ȫ���Ҳ�����װ����ԭ�п����Բⶨ�����Ӱ�죩��
3 CO2 + 6H2O + 8 Fe������ÿ����Ӧ����ȫ���Ҳ�����װ����ԭ�п����Բⶨ�����Ӱ�죩��
 �����NaOH��Һ����������ƿ���С��������֤��CO2��NaOH�����˷�Ӧ��
�����NaOH��Һ����������ƿ���С��������֤��CO2��NaOH�����˷�Ӧ��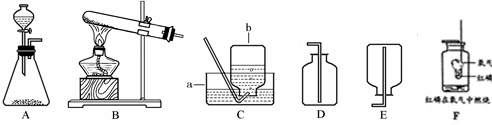




 �ĸ�������ʴ�����ϡ�
�ĸ�������ʴ�����ϡ�