��Ŀ����
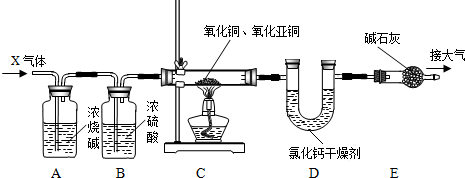
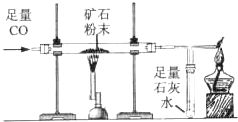 ������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�
������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�
��ʾ��FeCO3  ����FeO+CO2��
����FeO+CO2��
| ʵ��ǰ | ʵ��� | |
| Ӳ�ʲ����ܣ�����Ʒ�� | ����165.6g | ����159.6g |
| ˵���� | �ٿ�Ӳ�ʲ���������Ϊl45.6g �ں��������ʷ�Ӧ��ȫ | |
��2��ʵ�������ʢ�г���ʯ��ˮ���Թܵ��������ȷ�Ӧǰ������������______�ˣ�
�⣺��1��ʵ���з����Ļ�ѧ��Ӧ�У�FeCO3 FeO+CO2����CO+FeO
FeO+CO2����CO+FeO Fe+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��
Fe+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��
CO��ԭ���������ղ��������Ͷ�����̼��ʵ��ǰ��Ӳ�ʲ����ܣ�����Ʒ��������Ϊ165.6g-159.6g=6g��ʵ���Ͼ���FeCO3��ʧȥCO32-���������ɴ˿ɼ����FeCO3������Ϊ11.6g��ʵ��ǰӲ�ʲ����ܣ�����Ʒ������Ϊ165.6g����Ӳ�ʲ���������Ϊ145.6g���ó���Ʒ����Ϊ20g���ʺ�������Ŀ�ʯ��Ʒ��̼����������������Ϊ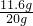 ��100%=58%
��100%=58%
���ԣ����Ӳ�ʲ�����������l45.6g��ʵ��ǰӲ�ʲ����ܣ�����Ʒ����������165.6g��ʵ���Ӳ�ʲ����ܣ�����Ʒ����������159.6g���μӷ�Ӧ����Ʒ��������165.6g-l45.6g=20g�����������ʵ���������Ϊ165.6g-159.6g=6g����̼���������Ϊ6g����̼������������Ϊx��
FeCO3��CO3
116 60
x 6g
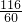 =
=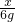
x=11.6g
̼����������Ʒ�е���������Ϊ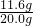 ��100%=58%
��100%=58%
��2��������֪̼���������ֻ��һ����ԭ�Ӻ�һ����̼�����ɶ�����̼����ʣ�ಿ��ֱ��ת��Ϊ������̼������һ����̼��Ӧ���ɵĶ�����̼������Ϊy����̼�������ֽ������ɵĶ�����̼����Ϊz��
CO3��һ����̼ת����CO2��̼�������ֽ������CO2
60 44 44
6g y z
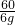 =
=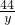 =
=
y=4.4g
z=4.4g
���Զ�����̼������Ϊ4.4g+4.4g=8.8g��
�ʴ�Ϊ��58%��
��2�����ɶ�����̼�������ǣ�8.8g����Щ������̼���Թ��еij���ʯ��ˮ���գ�ʵ�������ʢ�г���ʯ��ˮ���Թܵ��������ȷ�Ӧǰ������������ 8.8g��
�ʴ�Ϊ��8.8g��
��������1���������ʵļ�����Ҫ����Ϊ��̼�������������ʧȥ��CO3�����������ļ���ΪCO3�����������������ߵĹ�ϵ����̼���������������Ӷ������������������
��2����Ӳ�ʲ������м��ٵ�����������һ����̼��ϵ���Ԫ�ص�����������Ҫ������һ����̼��ϵ���Ԫ�ص��������������̼��������
������������Ҫ�����뻯ѧ��Ӧʽ�йصļ��㣬��һ�����Ѷȣ���Ҫͬѧ��ϸ�ķ������ô��⣮
 FeO+CO2����CO+FeO
FeO+CO2����CO+FeO Fe+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��
Fe+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��CO��ԭ���������ղ��������Ͷ�����̼��ʵ��ǰ��Ӳ�ʲ����ܣ�����Ʒ��������Ϊ165.6g-159.6g=6g��ʵ���Ͼ���FeCO3��ʧȥCO32-���������ɴ˿ɼ����FeCO3������Ϊ11.6g��ʵ��ǰӲ�ʲ����ܣ�����Ʒ������Ϊ165.6g����Ӳ�ʲ���������Ϊ145.6g���ó���Ʒ����Ϊ20g���ʺ�������Ŀ�ʯ��Ʒ��̼����������������Ϊ
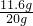 ��100%=58%
��100%=58%���ԣ����Ӳ�ʲ�����������l45.6g��ʵ��ǰӲ�ʲ����ܣ�����Ʒ����������165.6g��ʵ���Ӳ�ʲ����ܣ�����Ʒ����������159.6g���μӷ�Ӧ����Ʒ��������165.6g-l45.6g=20g�����������ʵ���������Ϊ165.6g-159.6g=6g����̼���������Ϊ6g����̼������������Ϊx��
FeCO3��CO3
116 60
x 6g
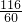 =
=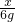
x=11.6g
̼����������Ʒ�е���������Ϊ
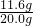 ��100%=58%
��100%=58%��2��������֪̼���������ֻ��һ����ԭ�Ӻ�һ����̼�����ɶ�����̼����ʣ�ಿ��ֱ��ת��Ϊ������̼������һ����̼��Ӧ���ɵĶ�����̼������Ϊy����̼�������ֽ������ɵĶ�����̼����Ϊz��
CO3��һ����̼ת����CO2��̼�������ֽ������CO2
60 44 44
6g y z
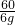 =
=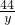 =
=
y=4.4g
z=4.4g
���Զ�����̼������Ϊ4.4g+4.4g=8.8g��
�ʴ�Ϊ��58%��
��2�����ɶ�����̼�������ǣ�8.8g����Щ������̼���Թ��еij���ʯ��ˮ���գ�ʵ�������ʢ�г���ʯ��ˮ���Թܵ��������ȷ�Ӧǰ������������ 8.8g��
�ʴ�Ϊ��8.8g��
��������1���������ʵļ�����Ҫ����Ϊ��̼�������������ʧȥ��CO3�����������ļ���ΪCO3�����������������ߵĹ�ϵ����̼���������������Ӷ������������������
��2����Ӳ�ʲ������м��ٵ�����������һ����̼��ϵ���Ԫ�ص�����������Ҫ������һ����̼��ϵ���Ԫ�ص��������������̼��������
������������Ҫ�����뻯ѧ��Ӧʽ�йصļ��㣬��һ�����Ѷȣ���Ҫͬѧ��ϸ�ķ������ô��⣮

��ϰ��ϵ�д�
�����Ŀ
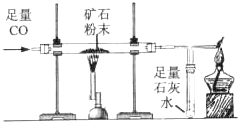 ������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У�
������ͼ��ʾװ�òⶨij��������Ŀ�ʯ��Ʒ��̼���������������������ʲ�����Ԫ������ʵ������в������κα仯����ʵ�����ݼ�¼���±��У���ʾ��FeCO3
| ||
| ʵ��ǰ | ʵ��� | |
| Ӳ�ʲ����ܣ�����Ʒ�� | 165.6g | 159.6g |
| ˵ �� | �ٿ�Ӳ�ʲ���������Ϊl45.6g �ں��������ʷ�Ӧ��ȫ | |
��2��ʵ�������ʢ�г���ʯ��ˮ���Թܵ��������ȷ�Ӧǰ������������
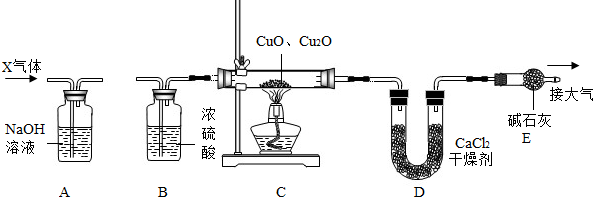
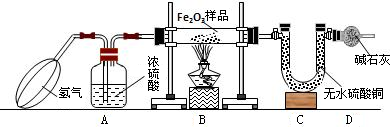
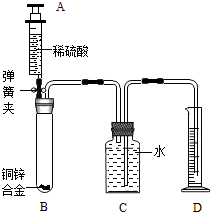 ��2012?�人��ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��
��2012?�人��ij��ѧС��������ͼ��ʾװ�òⶨͭп�Ͻ���Ʒ��п������������ͼ�й̶�װ������ȥ��