��Ŀ����
��ѧʵ��С����ʵ�����Ʊ�����ʱ������û��п������м������һЩ����Ƭ�������й�������ʾ�����ǽ���Ƭ�����ռ���Һ�У���ȥ���������Ĥ��Al2O3����ϴ����������ȡ�������ش��������⣺
��1����֪���������ռ���Һ��Ӧʱ��������ƫ�����ƣ�NaAlO2����ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ _________ ��
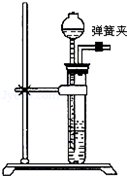
��2��ͼ����ʵ��С����װ����ȡ�����ķ���װ�ã������װ�������Եķ����� _______ ��
��1����֪���������ռ���Һ��Ӧʱ��������ƫ�����ƣ�NaAlO2����ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ _________ ��
��2��ͼ����ʵ��С����װ����ȡ�����ķ���װ�ã������װ�������Եķ����� _______ ��
��3���ý�������ϡ���ᷴӦ��ȡ�����Ļ�ѧ����ʽ�� _________ ��
��4���ռ������ij��÷����� _________ ��
��5������ͬ�¶��£�����пƬ����Ƭ�ֱ�����ͬŨ�ȵ����ᷴӦ��ȡ��������Ӧ���ʣ�п _________ �����������=������������������ _________ ��
��4���ռ������ij��÷����� _________ ��
��5������ͬ�¶��£�����пƬ����Ƭ�ֱ�����ͬŨ�ȵ����ᷴӦ��ȡ��������Ӧ���ʣ�п _________ �����������=������������������ _________ ��
��1��Al2O3+2NaOH==2NaAlO2+H2O
��2���رյ��ɼУ��ӳ���©��������עˮ����û����©���¶˹ܿڣ�����ע������ˮ����©�������γ�ˮ�������ã��۲�ˮ���Ƿ��½�����رյ��ɼУ��ӳ���©���л���עˮ����û����©���¶˹ܿڣ�����ˮ�����Թܣ��۲쳤��©�������Ƿ���Һ��������
��3��2Al+6HCl==2AlCl3+3H2��
��4�������ſ�����������ˮ����
��5����������п�������ǿ
��2���رյ��ɼУ��ӳ���©��������עˮ����û����©���¶˹ܿڣ�����ע������ˮ����©�������γ�ˮ�������ã��۲�ˮ���Ƿ��½�����رյ��ɼУ��ӳ���©���л���עˮ����û����©���¶˹ܿڣ�����ˮ�����Թܣ��۲쳤��©�������Ƿ���Һ��������
��3��2Al+6HCl==2AlCl3+3H2��
��4�������ſ�����������ˮ����
��5����������п�������ǿ

��ϰ��ϵ�д�
�����Ŀ
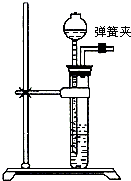 31����ѧʵ��С����ʵ�����Ʊ�����ʱ������û��п������м������һЩ����Ƭ�������й�������ʾ�����ǽ���Ƭ�����ռ���Һ�У���ȥ���������Ĥ��Al2O3����ϴ����������ȡ������
31����ѧʵ��С����ʵ�����Ʊ�����ʱ������û��п������м������һЩ����Ƭ�������й�������ʾ�����ǽ���Ƭ�����ռ���Һ�У���ȥ���������Ĥ��Al2O3����ϴ����������ȡ������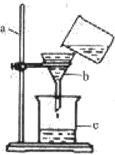 ij��ѧʵ��С����ʵ�����ж�һ�ձ����ǵĺ�ˮ�����˼������ش��������⣺
ij��ѧʵ��С����ʵ�����ж�һ�ձ����ǵĺ�ˮ�����˼������ش��������⣺