��Ŀ����
ij����ʯ����Ҫ�ɷ���������������������ɷ־�������Ԫ�غ���Ԫ�أ�����
�ⶨ������������������Ԫ�صĺ���Ϊ30%������ʯ����Ԫ�صĺ���Ϊ24%��
��1����������������Ļ�ѧʽ�� ��
��2������һ����̼��ԭ������ʯұ��������1 t�ÿ�ʯ����������96%��������������
���٣���Ҫ��д��������̣�����������С�����1λ��
�� Fe2O3 ��1�֣�
�ڲο�����������£�����������̺���������ȷ�÷֡�
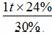 �۽������ʯ��Fe2O3������= = 0.8 t ��1�֣�
�۽������ʯ��Fe2O3������= = 0.8 t ��1�֣�
�裺����������Ϊx
Fe2O3 + 3CO  2Fe + 3CO2
2Fe + 3CO2
160 112
0.8 t 96% x
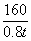 =
= 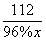
x= 0.6 t ��1�֣�
�����ɺ���96%������������Ϊ0.6 t��

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ








