题目内容
某同学为探究可燃物燃烧的条件,他查阅资料得知:白磷着火点为40℃,红磷着火点为240℃,它们在空气中燃烧都会生成刺激呼吸道的白烟--五氧化二磷,五氧化二磷易溶于水,并能与水反应,生成有毒的偏磷酸(HPO3).他按图所示装置进行对比实验:
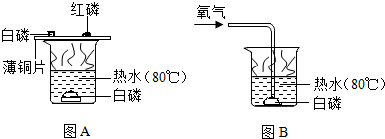
(1)用图A所示装置进行实验,观察到的现象是铜片上的白磷燃烧产生白烟,铜片上的红磷不燃烧,由此得出可燃物燃烧的条件是: ;再用图B所示装置进行实验,观察到:不通氧气时,热水中的白磷不燃烧,通氧气时,热水中的白磷燃烧,由此得出,可燃物燃烧的条件是 .
(2)根据燃烧的条件,选择填空:用扇子扇炉火不会熄灭,而是越扇越旺,原因是 ;用扇子扇蜡烛的烛火一扇就立即熄灭,原因是 (填序号).
A.可快速地提供燃烧所需的氧气,空气流动虽带走热量,但温度未降到着火点以下.
B.主要是空气流动带走热量,温度降到着火点以下.
(3)图A与图B所示实验相比,符合环保要求的是 .
(4)为探究实验后溶液的性质,在图B烧杯中滴入几滴石蕊试液,溶液呈红色,说明五氧化二磷与水反应所得溶液呈 ,五氧化二磷和水反应的化学方程式为 .

(1)用图A所示装置进行实验,观察到的现象是铜片上的白磷燃烧产生白烟,铜片上的红磷不燃烧,由此得出可燃物燃烧的条件是:
(2)根据燃烧的条件,选择填空:用扇子扇炉火不会熄灭,而是越扇越旺,原因是
A.可快速地提供燃烧所需的氧气,空气流动虽带走热量,但温度未降到着火点以下.
B.主要是空气流动带走热量,温度降到着火点以下.
(3)图A与图B所示实验相比,符合环保要求的是
(4)为探究实验后溶液的性质,在图B烧杯中滴入几滴石蕊试液,溶液呈红色,说明五氧化二磷与水反应所得溶液呈
考点:燃烧的条件与灭火原理探究,书写化学方程式、文字表达式、电离方程式
专题:科学探究
分析:(1)由图AB可知缺少氧气或温度未达到着火点物质不会燃烧
(2)从炉火温度较高产生的热量较多及补充氧气和蜡烛产生的热量较低的量去分析
(3)磷的燃烧会产生五氧化二磷白烟污染空气,从这一角度分析即可
(4)从石蕊与酸性溶液会变红这一规律解决
(2)从炉火温度较高产生的热量较多及补充氧气和蜡烛产生的热量较低的量去分析
(3)磷的燃烧会产生五氧化二磷白烟污染空气,从这一角度分析即可
(4)从石蕊与酸性溶液会变红这一规律解决
解答:解:(1)由图A知红磷未燃烧是因为温度没有达到其着火点,水下白磷未燃烧是因为缺乏氧气,B中温度达到了着火点未燃而通入氧气后燃烧说明氧气是燃烧的必要条件
故答案为:温度达到着火点;需要接触空气(或氧气);
(2)同样是扇子煽火结果不同,是因为炉火温度较高产生的热量较多煽走的热量不能是温度降到着火点以下,相反煽火的时候补充氧气,所以炉火会更旺,而蜡烛产生的热量较低煽火时温度降到了蜡烛的着火点以下故蜡烛熄灭
故答案为:A;B;
(3)磷的燃烧会产生五氧化二磷白烟污染空气,在水下反应可使白烟溶解于水中,防止了空气污染
故答案为:图B
(4)紫色的石蕊试液遇酸性溶液会变红;在图B烧杯中滴入几滴石蕊试液,溶液呈红色,说明五氧化二磷与水反应所得溶液呈酸性;五氧化二磷和水反应生成磷酸;
故答案为:酸性;P2O5+3H2O=2H3PO4.
故答案为:温度达到着火点;需要接触空气(或氧气);
(2)同样是扇子煽火结果不同,是因为炉火温度较高产生的热量较多煽走的热量不能是温度降到着火点以下,相反煽火的时候补充氧气,所以炉火会更旺,而蜡烛产生的热量较低煽火时温度降到了蜡烛的着火点以下故蜡烛熄灭
故答案为:A;B;
(3)磷的燃烧会产生五氧化二磷白烟污染空气,在水下反应可使白烟溶解于水中,防止了空气污染
故答案为:图B
(4)紫色的石蕊试液遇酸性溶液会变红;在图B烧杯中滴入几滴石蕊试液,溶液呈红色,说明五氧化二磷与水反应所得溶液呈酸性;五氧化二磷和水反应生成磷酸;
故答案为:酸性;P2O5+3H2O=2H3PO4.
点评:此题是对燃烧条件探究的拓展与延伸,能很好的训练学生的分析问题的能力,对加强学生对燃烧条件的认识大有好处

练习册系列答案
 世纪百通期末金卷系列答案
世纪百通期末金卷系列答案
相关题目
下列各组物质,能在pH=2的溶液中大量共存,且形成无色溶液的是( )
| A、NaNO3 BaCl2 Na2SO4 |
| B、Na2SO4 HCl KNO3 |
| C、CuSO4 NaCl NaNO3 |
| D、Na2CO3 NaOH Na2SO4 |
下列关于氢氧化钠的说法正确的是( )
| A、该固体物质能作干燥剂,可以干燥氮气、二氧化碳等气体 |
| B、该物质溶液的pH<7,可以用来除去铁锈 |
| C、该物质的溶液具有均一性、稳定性 |
| D、该固体物质溶解在水中,溶液的温度会降低 |
如图是某些食物的近似pH,呈碱性的是( )
A、 |
B、 |
C、 |
D、 |
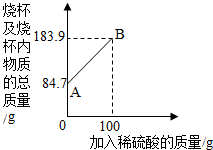 小华为测定某铁碳含金样品的成分,进行了如下实验:取一定质量的样品粉末于质量为61.7克的烧杯中,然后逐步加入一定质量分数的稀硫酸,当加入100g稀硫酸时反应恰好完全,加入稀硫酸的质量与烧杯及烧杯内物质的总质量的关系如图.
小华为测定某铁碳含金样品的成分,进行了如下实验:取一定质量的样品粉末于质量为61.7克的烧杯中,然后逐步加入一定质量分数的稀硫酸,当加入100g稀硫酸时反应恰好完全,加入稀硫酸的质量与烧杯及烧杯内物质的总质量的关系如图.