��Ŀ����
����Ŀ����ʯ�������������о���ʮ����Ҫ����;
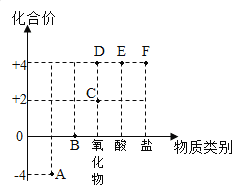
(1)��ʯ�ҽ���ˢǽ��,�����ǽ���Ӳ,��Ӧ�Ļ�ѧ����ʽ��_____
(2)����ʯ����������������,��Ӧ�Ļ�ѧ����ʽ��_____(�������к�������Ϊ��)
(3)����ʯ�ҷ����ľ��(��Ҫ�ɷ���K2CO3)��һ��������Ͽ��Ƶø�Ч����ũҩ���ڰ���ʹ��ʱ,ѡ������¶ˮ���糿,�����ڰ�������ֲ�ᆬҶ�Ͽ������ɼ����
�����ڰ�������ʯ�Ҹ���Ч,�����������˼��Ը�ǿ��KOH,��Ӧ�Ļ�ѧ����ʽ��_____
�����ڰ��������ṩֲ������Ӫ������_____(��Ԫ�ط���)
���𰸡�CO2+Ca(OH)2![]() CaCO3��+H2O Ca(OH)2+H2SO4=CaSO4+2H2O Ca(OH)2+K2CO3=CaCO3��+2KOH K
CaCO3��+H2O Ca(OH)2+H2SO4=CaSO4+2H2O Ca(OH)2+K2CO3=CaCO3��+2KOH K
��������
��1��ʯ�ҽ���������еĶ�����̼��Ӧ����̼��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��CO2+Ca��OH��2�TCaCO3��+H2O��
��2������ʯ�����������������������ᷴӦ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+H2SO4�TCaSO4+2H2O��
��3������ʯ�ҷ����ľ�ҷ�Ӧ����̼��Ƴ������������أ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+K2CO3�TCaCO3��+2KOH��
�ڡ��ڰۡ��к���ֲ�����������Ӫ��Ԫ�ؼ�Ԫ�أ��ڰۡ������ṩֲ������Ӫ������K��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ�������±��ش����⣺
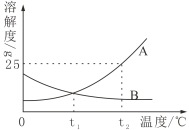
�¶�/�� | 20 | 40 | 60 | |
�ܽ�� | NaCl | 36.0 | 36.6 | 37.3 |
| 31.6 | 63.9 | 110 | |
(1)60����100gˮ��������ܽ�![]() ������Ϊ__________��
������Ϊ__________��
(2)����˵����ȷ����_________(�����)��
A.20��ʱ��![]() ������Һ����������Ϊ31.6%
������Һ����������Ϊ31.6%
B.40��ʱ��136.6gNaCl��Һ��һ������36.6gNaCl
C.��40��ʱ![]() �ı�����Һ���µ�20��������32.3g
�ı�����Һ���µ�20��������32.3g![]() ����
����
D.��40��ʱNaCl��![]() �ı�����Һ�ֱ�����20�������º�����Һ���������Ĵ�С��ϵΪNaCl��
�ı�����Һ�ֱ�����20�������º�����Һ���������Ĵ�С��ϵΪNaCl��![]()
(3)20��ʱ��������ͼʾ������
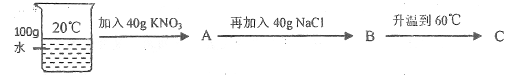
������ҺA��B��C�У�![]() �ܽ�ﵽ����״̬����_______(����ĸ)��
�ܽ�ﵽ����״̬����_______(����ĸ)��
���Ƚ���ҺA��B��![]() ������������A_______B(������� ������=��)��
������������A_______B(������� ������=��)��
����Ŀ������ʵ������ܴﵽʵ��Ŀ�ĵ��ǣ�������
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | �Ƚ�ͭ�����Ľ������ | ��ͭƬ����Ƭ��������������Һ�� |
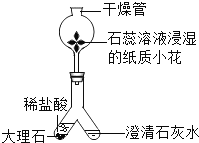
B | ������������ | �ֱ��ȼ�ӵ��ܷų������壬�ڻ����Ϸ�����һ���ڱ�Ϳ�г���ʯ��ˮ���ձ� |
C | �ⶨ | �ò�����պȡ��Һ�ε�ʪ���pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ������ |
D | ��ȥ | ��������建��ͨ��װ�� |
A. AB. BC. CD. D