��Ŀ����
��ѧ�κ�ѧ��ȤС���ͬѧ������ʵ����ʱ��������һƿ����������Һû������Ƥ����������ʦͬ���չ������̽����
�������1 ������������Һ�Ƿ�������أ�
ʵ��̽��1
| ʵ����� | ʵ������ | ʵ����� |
| ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ______ | ����������Һһ�������ˣ� |
���������
����1������������Һ���ֱ��ʣ�
����2������������Һȫ�����ʣ�
�������� �Ȼ�����Һ�����ԣ�
ʵ��̽��2
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | ��______���ɣ� | ˵��ԭ��Һ��һ����̼���ƣ� ��Ӧ����ʽ______ |
| ��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ����______ |
��˼�����ۣ�
��1������������Һ¶���ڿ��������ױ��ʣ���д����ط�Ӧ�Ļ�ѧ����ʽ��______��
��2��������[ʵ��̽��2]�У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷���______������С������С�����
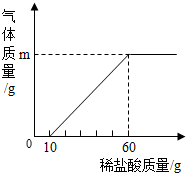
�������3������Һ�ı��ʳ̶�����أ�
ʵ�鲽�裺ȡ����Һ50�ˣ���μ�����������Ϊ7.3%��ϡ������������ð��Ϊֹ�����������������������������ϵ��ͼ��
�Լ��㣺
��1��m=______g��
��2��ԭ��Һ���������Ƶ����������Ƕ��٣�
�⣺�������1��
ʵ��̽��1����֪����������Һ�ѱ��ʣ���������������Һ�к���̼������ӣ���˼�������ز���������̼���壬�ʼ�������������ݲ�����
�������2��
ʵ��̽��2����1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ����̼�������Ȼ���������̼�ᱵ�����������а�ɫ�������ɣ�˵��ԭ��Һ��һ����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+BaCl2�T2NaCl+BaCO3����
��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ����Һ���ɫ����Һ�Լ��ԣ�����Һ��̼�����ѱ��Ȼ�����ȫ���ģ�����˵��ԭ��Һ��һ�����������ƣ��Ӷ��ó�[ʵ�����]������������Һ���ֱ��ʣ�
��˼�����ۣ�
��1������������Һ¶���ڿ����������������̼��Ӧ����̼���ƺ�ˮ����ط�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O��
��2����������������Һ�����Ȼ�����Һ�Dz��еģ���Ϊ����������̼���Ʒ�Ӧ�������������ƣ�������ȷ����2�������ʵ����ۣ��ò����������Ʋ��ֱ��ʵĽ��ۣ����Ը÷��������У�
�������3����1�����������֪�����ĵ����������ʵ�����Ϊ����60g-10g����7.3%=3.65g��
��ԭ��Һ��̼���Ƶ�����Ϊx�����ɶ�����̼������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x 3.65g y
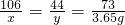
��ã�x=5.3g��y=2.2g
��m��ֵΪ2.2
��2��ԭ��Һ��̼���Ƶ���������Ϊ�� ��100%=10.6%��
��100%=10.6%��
�����������Ƶ���������Ϊ��1-10.6%=89.4%
�ʴ�Ϊ��
�������1��
ʵ��̽��1����������
ʵ��̽��2����ɫ��������������
ʵ����ۣ�����
��˼�����ۣ���1��CO2+2NaOH�TNa2CO3+H2O��
��2��������
�������3����1��2.2��
��2��89.4%��
�������������1��
ʵ��̽��1����֪����������Һ�ѱ��ʣ���������������Һ��һ������̼������ӣ���Ϊ����������Һ�����տ����еĶ�����̼��������Ӧ����̼���ƣ��ʼ�������������ݲ�����
�������2��
ʵ��̽��2����1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ����Һ�е�̼���ƻ���֮��Ӧ����̼��ư�ɫ������
��2���ӣ�1���Ľ��ۿ�֪ԭ��Һ�к���̼����ʱ���μ��Ȼ�����Һʱһ�������ɰ�ɫ��̼�ᱵ������ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ����Һ���ɫ����̼��������ȫ���Ȼ�����Ӧ������˵��ԭ��Һ��һ�����������ƣ�
ʵ����ۣ�������������Һ���ֱ��ʣ�
��˼�����ۣ���1������������Һ¶���ڿ��������ױ��ʣ���Ҫ�����������������̼��Ӧ����̼���ƺ�ˮ���µģ����Ծݴ�д���÷�Ӧ�Ļ�ѧ����ʽ��
��2��������[ʵ��̽��2]�У���������������Һ�����Ȼ�����Һ�Dz��еģ���Ϊ����������̼���Ʒ�Ӧ�������������ƣ�������ȷ��ԭ��Һ�Ƿ����������ƣ���˵ò����������Ʋ��ֱ��ʵĽ��ۣ����Ծݴ˽����⣮
�������3������������Ϣ����֪�����������������Ȼ����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ���Լ����̼���Ƶ�����������������������Ƶ�����������
������������һ��ʵ��̽���⣬����Ĺؼ��ǰ�����֤���������Ʋ��ֱ���ʱ���ȳ�ȥ���������е�̼���ƣ�Ȼ����֤��ʣ����Һ�Լ��ԣ�����˵���������Ʋ��ֱ��ʣ�
ʵ��̽��1����֪����������Һ�ѱ��ʣ���������������Һ�к���̼������ӣ���˼�������ز���������̼���壬�ʼ�������������ݲ�����
�������2��
ʵ��̽��2����1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ����̼�������Ȼ���������̼�ᱵ�����������а�ɫ�������ɣ�˵��ԭ��Һ��һ����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+BaCl2�T2NaCl+BaCO3����
��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ����Һ���ɫ����Һ�Լ��ԣ�����Һ��̼�����ѱ��Ȼ�����ȫ���ģ�����˵��ԭ��Һ��һ�����������ƣ��Ӷ��ó�[ʵ�����]������������Һ���ֱ��ʣ�
��˼�����ۣ�
��1������������Һ¶���ڿ����������������̼��Ӧ����̼���ƺ�ˮ����ط�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O��
��2����������������Һ�����Ȼ�����Һ�Dz��еģ���Ϊ����������̼���Ʒ�Ӧ�������������ƣ�������ȷ����2�������ʵ����ۣ��ò����������Ʋ��ֱ��ʵĽ��ۣ����Ը÷��������У�
�������3����1�����������֪�����ĵ����������ʵ�����Ϊ����60g-10g����7.3%=3.65g��
��ԭ��Һ��̼���Ƶ�����Ϊx�����ɶ�����̼������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x 3.65g y
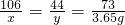
��ã�x=5.3g��y=2.2g
��m��ֵΪ2.2
��2��ԭ��Һ��̼���Ƶ���������Ϊ��
 ��100%=10.6%��
��100%=10.6%�������������Ƶ���������Ϊ��1-10.6%=89.4%
�ʴ�Ϊ��
�������1��
ʵ��̽��1����������
ʵ��̽��2����ɫ��������������
ʵ����ۣ�����
��˼�����ۣ���1��CO2+2NaOH�TNa2CO3+H2O��
��2��������
�������3����1��2.2��
��2��89.4%��
�������������1��
ʵ��̽��1����֪����������Һ�ѱ��ʣ���������������Һ��һ������̼������ӣ���Ϊ����������Һ�����տ����еĶ�����̼��������Ӧ����̼���ƣ��ʼ�������������ݲ�����
�������2��
ʵ��̽��2����1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ����Һ�е�̼���ƻ���֮��Ӧ����̼��ư�ɫ������
��2���ӣ�1���Ľ��ۿ�֪ԭ��Һ�к���̼����ʱ���μ��Ȼ�����Һʱһ�������ɰ�ɫ��̼�ᱵ������ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ����Һ���ɫ����̼��������ȫ���Ȼ�����Ӧ������˵��ԭ��Һ��һ�����������ƣ�
ʵ����ۣ�������������Һ���ֱ��ʣ�
��˼�����ۣ���1������������Һ¶���ڿ��������ױ��ʣ���Ҫ�����������������̼��Ӧ����̼���ƺ�ˮ���µģ����Ծݴ�д���÷�Ӧ�Ļ�ѧ����ʽ��
��2��������[ʵ��̽��2]�У���������������Һ�����Ȼ�����Һ�Dz��еģ���Ϊ����������̼���Ʒ�Ӧ�������������ƣ�������ȷ��ԭ��Һ�Ƿ����������ƣ���˵ò����������Ʋ��ֱ��ʵĽ��ۣ����Ծݴ˽����⣮
�������3������������Ϣ����֪�����������������Ȼ����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ���Լ����̼���Ƶ�����������������������Ƶ�����������
������������һ��ʵ��̽���⣬����Ĺؼ��ǰ�����֤���������Ʋ��ֱ���ʱ���ȳ�ȥ���������е�̼���ƣ�Ȼ����֤��ʣ����Һ�Լ��ԣ�����˵���������Ʋ��ֱ��ʣ�

��ϰ��ϵ�д�
�����Ŀ
