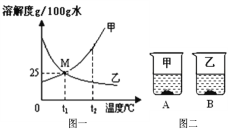
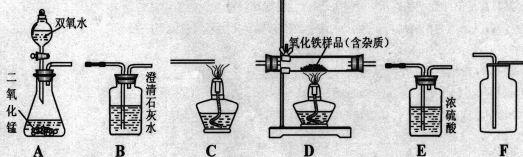
��Ŀ����
����Ŀ����ʵġ�̼�������˵����������һ���߽���̼�������硣
��1������л����� ��д��ѧʽ�����ɱ��������˹����꣬�仯ѧʽ�� ��
��2��CO��CO2�Ļ�ѧ���ʲ�ͬ������Ϊ���� ��ͬ��
��3��̼Ԫ�ص�ͬ���������н��ʯ��ʯī�� �����п���������Ǧ��о���� ��
��4��������̼��һ�ֱ������Դ���ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ �������� Ԫ�ص�����������ߣ�1mol��������Ԫ�ص����ʵ����� ��
���𰸡���1��CH4��CO2 ��2������������ӹ��ɡ����ӽṹ������������ ��3��C60����̼60�� ��ʯī�� ��4��CO2+2NH3 ![]() CO��NH2)2+H2O�� ������N����4mol
CO��NH2)2+H2O�� ������N����4mol
��������
�����������1������л�����CH4���ɱ��������˹����꣬�仯ѧʽ��CO2����2��CO��CO2�Ļ�ѧ���ʲ�ͬ������Ϊ���߷��ӽṹ��ͬ����3��̼Ԫ�ص�ͬ���������н��ʯ��ʯī��C60�����п���������Ǧ��о����ʯī����4��������̼��һ�ֱ������Դ���ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪCO2+2NH3 ![]() CO��NH2)2+H2O���������и������ԭ�������Ĵ�С��֪��Ԫ�ص�����������ߣ�1mol��������Ԫ�ص����ʵ�����4mol
CO��NH2)2+H2O���������и������ԭ�������Ĵ�С��֪��Ԫ�ص�����������ߣ�1mol��������Ԫ�ص����ʵ�����4mol