��Ŀ����
����Ŀ������������������������Ӧ�ù㷺��
��1����ͭ��ͭ��п�Ͻ�����˵���в���ȷ���� ______ ��
A ��ͭ���ڴ����� ![]() ��ͭ���ڽ�������
��ͭ���ڽ�������
C ��ͭ��Ӳ�ȱ�ͭ�� ![]() ��ͭ�Ŀ���ʴ���ܸ���
��ͭ�Ŀ���ʴ���ܸ���
��2��ͭ��������ͭ˿���������ý����� ______ ��
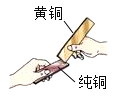
��3����ͭ��ͭп�Ͻ𣬽���ͭƬ�ͻ�ͭƬ����̻�����ͼ��ʾ������ͭƬ���������ԵĻ��ۣ���ʵ��˵���� ______ ��
��4��ͭ���ڳ�ʪ�Ŀ�����Ҳ�������⣬ͭ�⣨�׳�ͭ�̣�����Ҫ�ɷ���![]() ���������ɷ�����ͭ������ͭ�������� ______ �� ______ ��ͬ���õĽ����ͭ����ͭ��Ļ�ѧ����ʽΪ ______ ��
���������ɷ�����ͭ������ͭ�������� ______ �� ______ ��ͬ���õĽ����ͭ����ͭ��Ļ�ѧ����ʽΪ ______ ��
��5��������һЩ���������������������£�
�������� | ͭ | �� | п | �� | Ǧ |
�����ԣ������ĵ�����Ϊ100������ | 99 | 61 | 27 | 17 |
|
�ܶ� |
|
|
|
|
|
�۵� | 1083 | 660 | 419 | 1535 | 328 |
Ӳ�ȣ��Խ��ʯ��Ӳ��Ϊ10������ |
|
|
|
|
|
�����������ݣ���ʯ�õĴ������ƶ��������ƣ�ԭ���� ______ ��
���𰸡�A ��չ�� ��ͭ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ� ˮ ������̼ 2Cu+O2+H2O+CO2=Cu2��OH��2CO3 ����Ӳ�ȱ�����
��������
��1���Ͻ���ָ��һ�ֽ����м����ۺ�����������ǽ������γɵľ��н������Ե����ʣ���˺Ͻ��ǻ����Ͻ�������ǣ��Ͻ��Ӳ�ȴ��۵�ͣ���ʴ���ʴ�Ϊ��A��
��2��ͭ��������ͭ˿���������ý�������չ�ԣ�
��3������ͭƬ�ͻ�ͭƬ����̻�����ͭƬ���������ԵĻ��ۣ�˵����ͭ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ�
��4����ѧ��Ӧǰ��Ԫ������䣬��ʽ̼��ͭ�к���̼Ԫ�غ���Ԫ�أ�����ͭ����ʱһ���к���̼Ԫ�غ���Ԫ�ص����ʲμӣ������к�̼�������Ƕ�����̼������Ԫ�ص�������ˮ������ͭ������ͭ��������ˮ��������̼��ͬ���õĽ����ͭ����ͭ��Ļ�ѧ����ʽΪ��2Cu+O2+H2O+CO2=Cu2��OH��2CO3
��5������Ӳ�ȱ�������ʯ�õĴ���������Ķ��������Ƶġ�
�ʴ�Ϊ��
��1��A��
��2����չ�ԣ�
��3����ͭ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ�
��4��ˮ��������̼��2Cu+O2+H2O+CO2=Cu2��OH��2CO3��
��5������Ӳ�ȴ�

����Ŀ��������з�Ӧ�Ļ�ѧ����ʽ
1 | H2SO4 + Ca(OH)2 __________ | 2 | H2SO4 +Cu(OH)2__________ |
3 | H2SO4 + Fe(OH)3__________ | 4 | HCl + NaOH __________ |
5 | H2SO4 + NaOH__________ | 6 | Fe2O3 +HCl __________ |
7 | Fe2O3 + H2SO4__________ | 8 | CuO + HCl __________ |
9 | CuO + H2SO4__________ | 10 | HCl + CaCO3 __________ |