��Ŀ����
ij����С���ͬѧ��һ����̼��ͭ����Ʒ����ͭ�����������IJⶨ��������ͼ��ʾ��װ�ý���ʵ�飨���������ã�ͼ������̨����������ȥ����
�Իش��������⣺
��1�������ٵ�������______����������______��
��2��װ��D��������______��
��3����֪Ӳ�ʲ����ܢ���װ��ͭ��̼�Ļ����4g����Ӧ��ȫ��������������ֹͣ���ȣ���ȴ��Ƶ�Cװ������2.2g������Ʒ��ͭ������������______��
��4��ʵ����ɺ�ʦ����˵��������ʵ����ƣ���ʹ���з�Ӧ��ȫ��C��������ȫ��Ҳ����ó�ȷ�Ľ���������ۣ���ͬѧ�����A��B֮���һװ�ã��ٴ�ʵ��õ��˽�ȷ�Ľ���������жϣ�ԭ��ʵ���õ�ͭ������������______���ƫ�������䡱����ƫС������ԭ����______��
���𰸡�����������װ����ÿ��װ�õ����ã�Aװ�ò�������������������ͨ��Bװ���У�̼��ͭ�ֱ���������Ӧ���ɶ�����̼������ͭ��������̼��C�м�ʯ�����գ����ص�������Ϊ������̼��������D�м�ʯ����Ϊ�˷�ֹ�����еĶ�����̼��ˮ����C�б���ʯ��ˮ���գ�Ӱ��ʵ�����������������к���ˮ����������C�ᱻ���գ�
����⣺��1�������ٵ���������ƿ���������Dz�����
��2��Ϊ��ֹ����������Cװ�ñ���ʯ�����գ�Ӱ��ʵ��������Dװ�õ����������տ����еĶ�����̼��ˮ��������ֹ����Cװ��Ӱ��ʵ������
��3�����������غ㶨�ɣ���Ӧ���ɵĶ�����̼������Ϊװ��C���ص�������
ͬ���������̼��������Ϊ������̼��̼���������� 2.2g×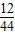 =0.6g
=0.6g
����ͭ������Ϊ4g-0.6g=3.4g
��ͭ����������Ϊ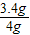 ×100%=85%
×100%=85%
��4����Ϊװ��B�������ݳ�ʱҪЯ��ˮ������ˮ����ͨ��B���Ա�װ��C�ļ�ʯ�����գ�ʹCװ�����ص�����ƫ�Ӷ�̼����������ͭ������ƫС��ͭ����������ƫС������Ӧ��A��B֮���һ��ϴ��ƿ���Գ�ȥ�����е�ˮ������
�ʴ�Ϊ����1����ƿ���ڲ����ܣ���2�����տ����ж�����̼��ˮ����ֹ����Cװ��Ӱ��ʵ��������3��85%����4��˫��ˮ�ֽ����������������һ����ˮ������ͨ��C��D���գ���ʹm2��ֵ����ʹ��õ�ͭ����������ƫС
����������������ؽ��Ǹ���ʵ��Ŀ�ķ������װ���и���װ�õ����ã�����ʵ��˼·�������ʵ����
����⣺��1�������ٵ���������ƿ���������Dz�����
��2��Ϊ��ֹ����������Cװ�ñ���ʯ�����գ�Ӱ��ʵ��������Dװ�õ����������տ����еĶ�����̼��ˮ��������ֹ����Cװ��Ӱ��ʵ������
��3�����������غ㶨�ɣ���Ӧ���ɵĶ�����̼������Ϊװ��C���ص�������
ͬ���������̼��������Ϊ������̼��̼���������� 2.2g×
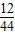 =0.6g
=0.6g����ͭ������Ϊ4g-0.6g=3.4g
��ͭ����������Ϊ
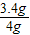 ×100%=85%
×100%=85%��4����Ϊװ��B�������ݳ�ʱҪЯ��ˮ������ˮ����ͨ��B���Ա�װ��C�ļ�ʯ�����գ�ʹCװ�����ص�����ƫ�Ӷ�̼����������ͭ������ƫС��ͭ����������ƫС������Ӧ��A��B֮���һ��ϴ��ƿ���Գ�ȥ�����е�ˮ������
�ʴ�Ϊ����1����ƿ���ڲ����ܣ���2�����տ����ж�����̼��ˮ����ֹ����Cװ��Ӱ��ʵ��������3��85%����4��˫��ˮ�ֽ����������������һ����ˮ������ͨ��C��D���գ���ʹm2��ֵ����ʹ��õ�ͭ����������ƫС
����������������ؽ��Ǹ���ʵ��Ŀ�ķ������װ���и���װ�õ����ã�����ʵ��˼·�������ʵ����

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
ij����С���ͬѧ��������̿�ۺ�16������ͭ���Ȼ�ϣ�����ͼ��ʾװ�ý���ʵ�飮ͼ������̨��װ������ȥ����ش��й����⣺
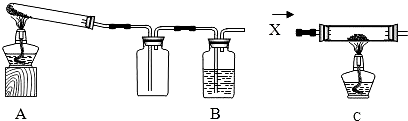
��1��ͬѧ����ͨ���ⶨ����̼����Ԫ�ص����������ɶ�����̼����������������ͭ��̿�۷�Ӧ�����������CO2���Ƿ������������Ӧһ��ʱ���ֹͣ���ȣ���ȴ�����£���Ӧǰ�����õ��������£�
�������ݷ��֣���Ӧ������̼����Ԫ�ص����� ������ڡ�����С�ڡ����ڡ������ɶ�����̼������������4���и���һ����йص����ļ�� ������д��ĸ��
A��װ���л���һ����CO2δ��ʯ��ˮ��Һ����
B������ͭ��̿�۷�Ӧ�����������CO2���CO
C������ͭ��̿��û����ȫ��Ӧ
D���÷�Ӧ�����������غ㶨��
��2����ͬѧ��Ϊ��ͼʾװ��C���������װ��A������ǰ��ͨһ������X��ֹͣ���Ⱥ���ͨһ������壬������ʹʵ���õ����ݸ���ȷ��˵�����⣮����Ϊ��O2��N2��H2���������У�XӦѡ����һ�����壿 ��
��3��ʵ��������ܵõ�ͭ���ٿˣ���д��������̣�

��1��ͬѧ����ͨ���ⶨ����̼����Ԫ�ص����������ɶ�����̼����������������ͭ��̿�۷�Ӧ�����������CO2���Ƿ������������Ӧһ��ʱ���ֹͣ���ȣ���ȴ�����£���Ӧǰ�����õ��������£�
| װ�� | ��Ӧǰ | ��Ӧ�� |
| A | �Թܵ�����38.2 �� ����ͭ��̿�ۻ���������20.0�� |
�Թܺ������ʵ�����56.8 �� |
| B | ��Ӧ��ƿ��ʯ��ˮ�ȷ�Ӧǰ����1.1 �� | |
A��װ���л���һ����CO2δ��ʯ��ˮ��Һ����
B������ͭ��̿�۷�Ӧ�����������CO2���CO
C������ͭ��̿��û����ȫ��Ӧ
D���÷�Ӧ�����������غ㶨��
��2����ͬѧ��Ϊ��ͼʾװ��C���������װ��A������ǰ��ͨһ������X��ֹͣ���Ⱥ���ͨһ������壬������ʹʵ���õ����ݸ���ȷ��˵�����⣮����Ϊ��O2��N2��H2���������У�XӦѡ����һ�����壿
��3��ʵ��������ܵõ�ͭ���ٿˣ���д��������̣�

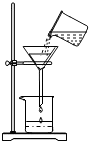 ��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺
��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺