��Ŀ����
ij����С���ͬѧ��ʯ�ҳ�����һ�麬���ʵ���ʯ������ȡ�Ȼ��ƣ����ȳƵ�����������Ϊ14g��Ȼ�������ʯ�ҷ����ձ��У������ձ��м���146g��������Ϊ10%��ϡ���ᣬǡ����ȫ��Ӧ�����ʲ���Ӧ��Ҳ������ˮ�����ѷ�Ӧ���Һ����ˣ���ֽ�����µĹ������ʾ�ϴ�ӡ������Ƶ���������Ϊ2.8g����������Ǽ��㣺��1�������ʯ�ҵĴ��ȣ�
��2���ܹ��Ƶ��Ȼ��ƶ��ٿˣ�
��������1���������ʲ���ϡ���ᷴӦ��ֻ�������ƺ�ϡ���ᷴӦ�����Կɸ��ݷ�Ӧ��ʣ��������������������Ƶ�������������������Ƶ�����������
��2��146g��������Ϊ10%��ϡ����ǡ������������ȫ��Ӧ���ɸ��ݻ�ѧ����ʽ���������Ȼ��Ƶ�������
��2��146g��������Ϊ10%��ϡ����ǡ������������ȫ��Ӧ���ɸ��ݻ�ѧ����ʽ���������Ȼ��Ƶ�������
����ⷨһ���������Ȼ��Ƶ�����Ϊx
CaO������=14g-2.8g=11.2g
��ʯ�ҵĴ���=
��100%=80%
CaO+2HCl=CaCl2+H2O
56 111
11.2g x
=
��x=22.2g
�ⷨ������μӷ�Ӧ������������Ϊx�����ɵ��Ȼ�������Ϊy
CaO+2HCl�T�T�T�TCaCl2+H2O
56 73 111
x 146g��10% y
=
��x=11.2g
=
��y=22.2g
��ʯ�ҵĴ���=
��100%=80%
�𣺣�1�������ʯ�ҵĴ�����80%����2���ܹ��Ƶ��Ȼ���22.2g��
CaO������=14g-2.8g=11.2g
��ʯ�ҵĴ���=
| 11.2g |
| 14��g |
CaO+2HCl=CaCl2+H2O
56 111
11.2g x
| 56 |
| 11.2g |
| 111 |
| x |
�ⷨ������μӷ�Ӧ������������Ϊx�����ɵ��Ȼ�������Ϊy
CaO+2HCl�T�T�T�TCaCl2+H2O
56 73 111
x 146g��10% y
| 56 |
| x |
| 73 |
| 146g��10% |
| 73 |
| 146g��10% |
| 111 |
| y |
��ʯ�ҵĴ���=
| 11.2g |
| 14g |
�𣺣�1�������ʯ�ҵĴ�����80%����2���ܹ��Ƶ��Ȼ���22.2g��
�������������������������ͻ�ѧ����ʽ���ϵļ����⣬����������Ҫ��ȷд��������Ӧ�Ļ�ѧ����ʽ��Ȼ��Ҫ�ص����� �����ʼ�������ȣ�����һ��Ҫϸ��ȷ��

��ϰ��ϵ�д�
 ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
�����Ŀ
ij����С���ͬѧ��������̿�ۺ�16������ͭ���Ȼ�ϣ�����ͼ��ʾװ�ý���ʵ�飮ͼ������̨��װ������ȥ����ش��й����⣺
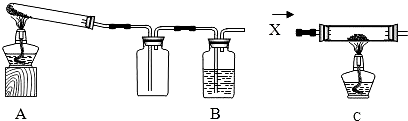
��1��ͬѧ����ͨ���ⶨ����̼����Ԫ�ص����������ɶ�����̼����������������ͭ��̿�۷�Ӧ�����������CO2���Ƿ������������Ӧһ��ʱ���ֹͣ���ȣ���ȴ�����£���Ӧǰ�����õ��������£�
�������ݷ��֣���Ӧ������̼����Ԫ�ص����� ������ڡ�����С�ڡ����ڡ������ɶ�����̼������������4���и���һ����йص����ļ�� ������д��ĸ��
A��װ���л���һ����CO2δ��ʯ��ˮ��Һ����
B������ͭ��̿�۷�Ӧ�����������CO2���CO
C������ͭ��̿��û����ȫ��Ӧ
D���÷�Ӧ�����������غ㶨��
��2����ͬѧ��Ϊ��ͼʾװ��C���������װ��A������ǰ��ͨһ������X��ֹͣ���Ⱥ���ͨһ������壬������ʹʵ���õ����ݸ���ȷ��˵�����⣮����Ϊ��O2��N2��H2���������У�XӦѡ����һ�����壿 ��
��3��ʵ��������ܵõ�ͭ���ٿˣ���д��������̣�

��1��ͬѧ����ͨ���ⶨ����̼����Ԫ�ص����������ɶ�����̼����������������ͭ��̿�۷�Ӧ�����������CO2���Ƿ������������Ӧһ��ʱ���ֹͣ���ȣ���ȴ�����£���Ӧǰ�����õ��������£�
| װ�� | ��Ӧǰ | ��Ӧ�� |
| A | �Թܵ�����38.2 �� ����ͭ��̿�ۻ���������20.0�� |
�Թܺ������ʵ�����56.8 �� |
| B | ��Ӧ��ƿ��ʯ��ˮ�ȷ�Ӧǰ����1.1 �� | |
A��װ���л���һ����CO2δ��ʯ��ˮ��Һ����
B������ͭ��̿�۷�Ӧ�����������CO2���CO
C������ͭ��̿��û����ȫ��Ӧ
D���÷�Ӧ�����������غ㶨��
��2����ͬѧ��Ϊ��ͼʾװ��C���������װ��A������ǰ��ͨһ������X��ֹͣ���Ⱥ���ͨһ������壬������ʹʵ���õ����ݸ���ȷ��˵�����⣮����Ϊ��O2��N2��H2���������У�XӦѡ����һ�����壿
��3��ʵ��������ܵõ�ͭ���ٿˣ���д��������̣�

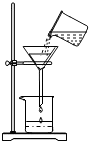 ��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺
��2012?������һģ��ij����С���ͬѧΪ�ⶨһƿBaCl2��Һ���ʵ�������������������ʵ�飬��ش��й����⣺