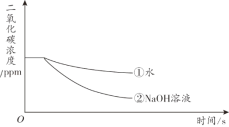
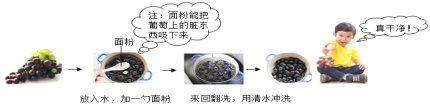
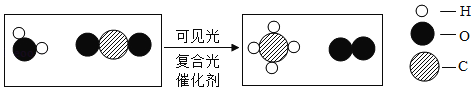
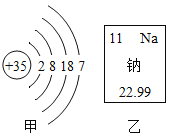
��Ŀ����
����Ŀ����ͼ��ʵ�����ü��ȸ�����صķ�����ȡ������װ��ͼ

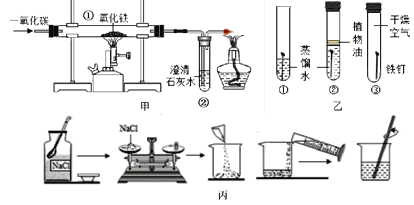
��1��д���б�ŵ����������ƣ�
��________����________����________����________��
��2����ʵ������ȡ����ʱ�������ܡ���Ƥ���ʹ��Թ����Ӻ��������Լ��ķ����ǣ���_______��������ܿ���____________����װ�ò�©����
���Թܿ���һ������������___________��
���Թܿ���������б��Ŀ����___________��
�ܳ��˿�������ˮ���ռ�����������������________���ռ�������
��3��д�����ȸ��������ȡ�����Ļ�ѧ����ʽ��________________��
���𰸡��Թ� �ƾ��� ����̨ ����ƿ ���ܷ���ˮ�У����ֽ����Թܱ� ����ð�� ��ֹ����ʱ������ط�ĩ���뵼�� ��ֹ����ˮ���������Թ�ը�� �����ſ��� ![]()
.
��������
��1���۲�װ��ͼ����������ֱ��Ǣ��Թܣ��ھƾ��ƣ�������̨���ܼ���ƿ��
��2���ټ�������Եķ����ǣ�������һ�˷���ˮ�У����ֽ����Թܱڣ������ܿ�������ð������װ�ò�©����
���Թܿ���һ��������Ϊ�˷�ֹ����ʱ������ط�ĩ���뵼�ܣ�ʹ���ܶ�����
���Թܿ���������б����Ϊ�˷�ֹ����ˮ���������Թ�ը�ѣ�
�������ܶȱȿ��������Ի����������ſ������ռ���
��3��������ؼ�����������ء��������̺���������Ӧ�Ļ�ѧ����ʽ�ǣ� ![]() ��
��

����Ŀ������������ͿƼ���չ�벻����ѧ����ش��������⣺
��1����������й���ͳ���գ��������ѳɴ�ͳ���ס����������ӵ�ԭ���У�Ŵ����������Ӫ������_____________��
��2����ȼ���м���������������ζ������C2H5SH��������ȼ��й©ʱ��ʱ���֣���ȼ�յĻ�ѧ����ʽΪ2C2H5SH+9O2![]() 4CO2+2X+6H2O����X�Ļ�ѧʽΪ_______��
4CO2+2X+6H2O����X�Ļ�ѧʽΪ_______��
��3���±��г��˼�ͥ��һЩ������Ʒ��pH��
��Ʒ | ʳ�� | ���� | ʳ����Һ | ����ˮ |
pH | 3 | 9 | 7 | 10 |
�Ʒ�Ĵ��Ǽ��Եģ����㱻�Ʒ���ˣ�Ӧ�����������е�_________ Ϳ��Ƥ���ϡ�