��Ŀ����
��һ�λ�ѧ�����У�С����С������ʯ����ˮ��Ӧ��ʵ��ʱ���������������ֲ�ͬ�����
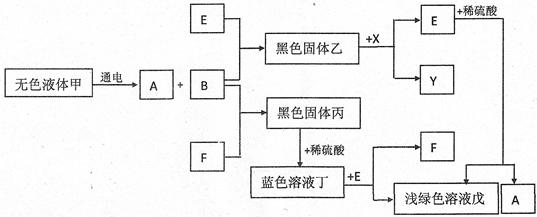
���Ǿ������������һ����Ϊ��С����ʵ����������쳣��������������õ���ʯ���Ѳ��ֱ��ʡ����Ƿֱ���Ʒ������±�������ʵ����֤��

������������ݣ��ش��������⣺
(1)����Ϊ˭�ķ�������ȷ?Ϊʲô? ��
(2)�û�ѧ����ʽ������ʯ�ұ��ʵ�ԭ�� ��
(3)���Լ�ƿ�Ĺ�����һ�����е������� ��
(4)ͨ������ʵ�飬����Ϊ������ʯ��ʱӦע�� ��

���Ǿ������������һ����Ϊ��С����ʵ����������쳣��������������õ���ʯ���Ѳ��ֱ��ʡ����Ƿֱ���Ʒ������±�������ʵ����֤��

������������ݣ��ش��������⣺
(1)����Ϊ˭�ķ�������ȷ?Ϊʲô? ��
(2)�û�ѧ����ʽ������ʯ�ұ��ʵ�ԭ�� ��
(3)���Լ�ƿ�Ĺ�����һ�����е������� ��
(4)ͨ������ʵ�飬����Ϊ������ʯ��ʱӦע�� ��
(1)С���ķ�������ȷ����ʯ����ˮ��Ӧ�����������ƣ�Ҳ��ʹ��̪��Һ��죬����֤���ѱ��� (2)CaO+H2O=Ca(OH)2 Ca(OH)2+CO2=CaCO3��+H2O (3) CaO��CaCO3��(4)�ܷⱣ��
����CaO��Ca(OH)2��CaCO3�����ʼ����ת���ǽ���Ĺؼ�����ʯ�ҵijɷ�ΪCaO�����к�ǿ����ˮ�ԣ�����������еĶ�����̼��Ӧ���ʡ�(1)С����ˮ�ܽ�ҩƷ���ټ����̪��Һ���÷�������ȷ����ʹ��û�б��ʵ���ʯ�Ҽ�ˮ��Ҳ����ˮ��Ӧ�����������ƶ�ʹ��̪��죻(3)С��ͬѧ����ƿ���Լ���ʵ�飬Ҳ�۲쵽��������ͷų�����������˵����ƿ�в�����CaO��С����ȡ������Ʒ�м����ᣬ�����ݲ�����˵����ƿ�л�����CaCO3��

��ϰ��ϵ�д�
�����Ŀ