��Ŀ����
����Ŀ��������֪,��֪�����ǻ�ѧ˼ά�����������ͼʾ�ش��������⣺

(1)�Ӻ��֪�ۣ�
��50mlˮ��50ml�Ҵ���Ϻ�,��Һ���С��100ml,�۽���Ϊ_____________________��
��ij����X��������ȼ�����ɵ�����ˮ,X������һ�����е�ԭ����____________(�����)
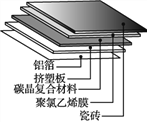
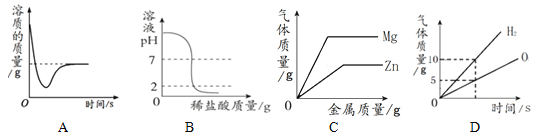
������ͼ1��ʾ�����š�Cu���ܱ�ʾ������Ϣ,���ʾͭԪ�ء�����ͭ����,���ܱ�ʾ___________��
�ܴ����ĽǶ�˵��ͼ2��Ӧ��ʵ����__________________________��
(2)����֪��ۣ�
����ͼ2,��A��B��C��D��E��,��Ӧ���ʻ�ѧ�������ȶ�����_____________���ѧʽ��,����ͬ��Ԫ�ص���___________(����)��C��Ԫ�����ڱ�λ�ڵ�_______���ڡ�
��ͼ4Ϊij��ѧ��Ӧ����ʾ��ͼ����÷�Ӧ�Ļ�ѧ����ʽΪ_________________��
���𰸡� ���Ӽ��м�� N��H һ��ͭԭ�� ��������̼������ӽ������ˮ�Ͷ�����̼ Ne CE �� C2H2+H2O![]() C2H4O
C2H4O
����������1�������ڷ��Ӽ��м����50mLˮ��50mL�Ҵ���Ϻ�һ�����Ҵ����Ӻ�ˮ�����ռ���˼����������Һ���С��100mL��
������X��������ȼ�����ɵ�����ˮ���������غ㶨�ɿ�֪��Ӧǰ��ԭ�ӵ�����䣬X������һ�����е�ԭ����N��H��
����Cu����ʾ������Ϣ�����ʾͭԪ�ء�����ͭ���ʣ����ܱ�ʾһ��ͭԭ�ӣ�
�ܴ�ͼ���Կ����÷�Ӧ��ʵ���������Ӻ�̼������ӽ������ˮ���ӺͶ�����̼���ӣ�
��2������A��B��C��D��E�У�B��ԭ�ӵ�������������8���ﵽ���ȶ��ṹ����Ӧ���ʻ�ѧ�������ȶ���B������Ϊ10��ΪNeԪ�أ�C��E����������ͬ������ͬһ��Ԫ�أ�����ͼ��֪��CΪþԪ�أ���ԭ�����������Ӳ㣬��λ��Ԫ�����ڱ��е������ڣ�
�������Ĺ��ɵĿ�֪���÷�Ӧ��C2H2��ˮ�ڸ�ѹ�����������·�Ӧ����C2H4O����Ӧ�Ļ�ѧ����ʽΪC2H2+H2O![]() C2H4O��
C2H4O��

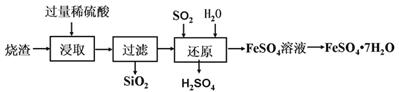
����Ŀ�������װ�г�ʹ��һ�ִ�װ��������Ʒ��Ϊ��504˫�����������ǩ��ͼ��ʾ��ij��ѧ��ȤС���һ�����õ���504˫������������Ʒ����Ũ�����Ȥ�����ʵ�����̽����

��������⡿�����ù���ijɷ���ʲô��
���������ϡ��������Ȼ�����Һ�ڳ����·�����Ӧ��Fe+2FeCl3=3FeCl2
�����װ�۲죺���ֹ���ʺ�ɫ�����ֹ���ʰ�ɫ��������������ɫ�Ŀ�״���塣
���������롿�����ù����п��ܺ���Fe��Fe2O3��CaO��Ca(OH)2��CaCO3��
���п��ܺ���Fe2O3��������________________________________��
��ʵ��̽��������ͬѧ�ķ�����
ʵ����� | ʵ������ | ʵ����� |
(1)ȡ�������ù������Թ��У��μ�����������ˮ������ | �����ܽ⣬�Թ���ڷ��� | ������һ������_________ |
(2)���ˣ�����Һ�еμ���ɫ��̪��Һ | ��Һ���ɫ | ������һ�������������� |
(3)ȡ���������Թ��У��μ�������______________ | ��������ʧ���д�����ɫ����������õ�dz��ɫ��Һ | ������һ������________�� һ������������ |
��ʵ�����ɡ�����ͬѧ��Ϊ��ͬѧ��ʵ���еó� ��һ���������������Ľ����Ǵ���ģ�������____________������Ϊ��ͬѧ��һ��ʵ������ó��Ľ���Ҳ��������____________��������______(�û�ѧ����ʽ��ʾ)��
������̽������ͬѧ��ȡ���õĹ������ʵ��̽����
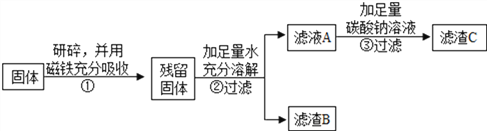
��ͬѧ������B����̽����
ʵ����� | ʵ������ | ʵ����� |
ȡ����B���Թ��У��μ�������ϡ���ᣬ��������ͨ�����ʯ��ˮ | ��������ʧ��______�� ������ð����___________ | ������һ������CaCO3��Fe2O3��д��Fe2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽ��________________ |
������ó����ۡ���
������ʵ�����̣���ͬѧ��ȡ5g���ù������飬�ô����������ò�������3g��������ˮ����ܽ���ˣ�������B������Ϊ1.8g������ҺA�м�����̼������Һ����˵�����C������Ϊ2g������㣺
(1)��ҺA�к��������Ƶ�����Ϊ______________��(д���������)
(2)ͨ�����㣬�ó����������ijɷ֣�__________________��(д���������)
����Ŀ��ij��ѧ��ȤС���ͬѧ��һ�ݹ�����Ʒ������̽����ͨ��ʵ����ȷ������Ʒ�������������ۻ�϶��ɡ����dz�ȡ��13.6g������Ʒ������ͼ��ʾ��װ�ü���ʵ�飬��ȫ��Ӧ�ⶨ���й��������±���ʾ��
A�й��� ������ | ����ʯ��ˮ ������ | |
��Ӧǰ | 13.6g | 102.5g |
��Ӧ�� | 11.2g | 108.0g |
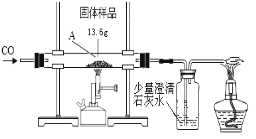
����㣺
��ʵ������ͨCO��Ŀ����____________________________________��
�ƴ��ϱ���ѡ����ʵ����ݣ����������Ʒ����������������_____________�ˡ�
����������Ӧ��Ĺ����м���100gϡ���ᣬǡ����ȫ��Ӧ����Ӧ��������Һ�����ʵ���������___������д������������̣������ȷ��0.1%��