��Ŀ����
A��K����Ba2+��Mg2+��Na+��Fe3+ ��OH-��Cl-��SO42-��CO32-��I��K�ǿ�������İ�ɫ������D�Dz�������İ�ɫ������F�Ǻ��ɫ����������Ϊ������ˮ�ļ���Σ�������ˮ��Һ�е�ת����ϵ����ͼ��ʾ����ش��������⣺

(1)д��B�Ļ�ѧʽ___________��
(2)A��J��Ӧ�Ļ�ѧ����ʽ__________________���䷴Ӧ����Ϊ_________��
(3)����C����ˮ����Һ���¶Ȼ�________ (����ߡ��������͡����䡱)��
(4)������ʾ�����������˿����Ա��ξͻᷢ���ж����˵�θҺ�ﺬ�����ᡱ�����û�ѧ����ʽ��ʾ���I���ж���ԭ��____________________________ ��
(2)A��J��Ӧ�Ļ�ѧ����ʽ__________________���䷴Ӧ����Ϊ_________��
(3)����C����ˮ����Һ���¶Ȼ�________ (����ߡ��������͡����䡱)��
(4)������ʾ�����������˿����Ա��ξͻᷢ���ж����˵�θҺ�ﺬ�����ᡱ�����û�ѧ����ʽ��ʾ���I���ж���ԭ��____________________________ ��
��1��Na2SO4
��2��Ba(OH)2+MgCl2=Mg(OH)2��+BaCl2�����ֽⷴӦ
��3������
��4��BaCO3+2HCl=BaCl2+H2O+CO2��
��2��Ba(OH)2+MgCl2=Mg(OH)2��+BaCl2�����ֽⷴӦ
��3������
��4��BaCO3+2HCl=BaCl2+H2O+CO2��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
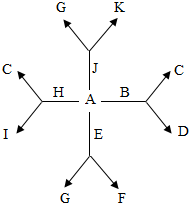 ��2013?��ҵ����ģ��A��K����Ba2+��Mg2+��Na+��Fe3+��OH-��Cl-��SO42-��CO32-���������е����ֹ��ɵij��������I��K�ǿ�������İ�ɫ������D�Dz�������İ�ɫ������F�Ǻ��ɫ����������Ϊ������ˮ�ļ���Σ�������ˮ��Һ�е�ת����ϵ��ͼ��ʾ����ش��������⣺
��2013?��ҵ����ģ��A��K����Ba2+��Mg2+��Na+��Fe3+��OH-��Cl-��SO42-��CO32-���������е����ֹ��ɵij��������I��K�ǿ�������İ�ɫ������D�Dz�������İ�ɫ������F�Ǻ��ɫ����������Ϊ������ˮ�ļ���Σ�������ˮ��Һ�е�ת����ϵ��ͼ��ʾ����ش��������⣺ ��2012?���죩A��K����Ba2+��Mg2+��Na+��Fe3+��OH-��Cl-��
��2012?���죩A��K����Ba2+��Mg2+��Na+��Fe3+��OH-��Cl-��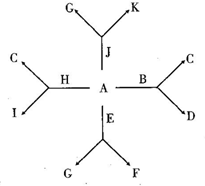 A��K����Ba2+��Mg2+��Na+��Fe3+��OH-��Cl-��
A��K����Ba2+��Mg2+��Na+��Fe3+��OH-��Cl-��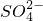 ��
��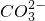 ���������е����ֹ��ɵij��������I��K�ǿ�������İ�ɫ������D�Dz�������İ�ɫ������F�Ǻ��ɫ����������Ϊ������ˮ�ļ���Σ�������ˮ��Һ�е�ת����ϵ��ͼ��ʾ����ش��������⣺
���������е����ֹ��ɵij��������I��K�ǿ�������İ�ɫ������D�Dz�������İ�ɫ������F�Ǻ��ɫ����������Ϊ������ˮ�ļ���Σ�������ˮ��Һ�е�ת����ϵ��ͼ��ʾ����ش��������⣺