��Ŀ����
A��B��C��DΪ���г��������ʣ�A��B֮�䷢���Ļ�ѧ��Ӧ�ɱ�ʾΪ����A+B��C+D����
��1����CΪ�Ȼ��ƣ�DΪ���������A��Һ�м��������ܲ�����������AΪ���� ����A��B����Һǡ����ȫ��Ӧ����Ӧ����Һ��pH���� 7�������������=������
��2����A��B��Ϊ��ɫ��ĩ��A��B��Ӧʱ�ɹ۲쵽��ɫ��ĩ��Ϊ��ɫ��ͬʱ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��A��B��Ӧ�Ļ�ѧ����ʽΪ���� ��
(1)���� = (2)C+2CuO 2Cu+CO2��
2Cu+CO2��
����

��ϰ��ϵ�д�
�����Ŀ
��3�֣������ͼʾ�ش����⣺
��1����������Һ��A������B�������������ʱ����
���ɼ��Թ��е�ˮ�����·��� ��
| A��ˮ������ | B������������Һ�Ͷ������� |
| C���ƾ��͵��� | D���Ȼ�����Һ��һ����̼ |
ijʵ��С�����÷�����Һ�Ʊ�K2SO4���о�CaSO4?2H2O���ȷֽ�IJ��
��һ��K2SO4���Ʊ�
��1����CaCO3�гɷ�ĩ��Ŀ���� ��
��2�����������п�ѭ��ʹ�õ�������CO2�� ����д��ѧʽ����
��3����Ӧ����������ʵ��ܽ�����±�������Ϊ��Ӧ���ڳ�������ʵ�ֵ�ԭ���� ��
| ���� | KCl | K2SO4 | NH4Cl | M |
| �ܽ��/g��25�棩 | 34.0 | 11.1 | 37.2 | 19.5 |
��4������ˮ���ñ���K2SO4��Һϴ�ӷ�Ӧ�����þ����Ŀ���� ��Ϊ����˾����Ƿ�ϴ�Ӹɾ�����ȡ���һ��ϴ��Һ���ȼ��� ��ѡ����ţ���ͬ���������ã������ϲ���Һ�еμ� ���۲������жϣ�
a��AgNO3��Һ b��������BaCl2��Һ c��������Ba��NO3��2��Һ
�������о�CaSO4?2H2O���ȷֽ�IJ��
��5���������õ�CaSO4?2H2O����CaCO3�����������ȥ���õĻ�ѧ��Ӧ����ʽ ��
��6��Ϊ�˲ⶨCaSO4?2H2O��CaCO3��������x��y��ʵ��С��������ͼ��ʾ��װ�ã��г�����ʡ�ԣ�����ʵ�飮
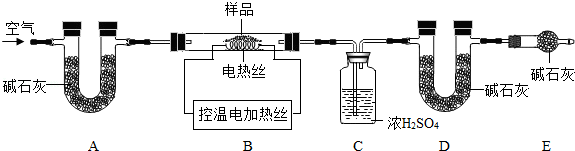
��ʵ��ǰ����Ҫ ����װ����Ʒ��װ��A�������� ��
����֪CaSO4?2H2O��160������CaSO4��1350��ʱCaSO4��ʼ�ֽ⣻CaCO3��900��ʱ�ֽ���ȫ���ֿ���Bװ���¶�900�����ʵ�鲢�ɼ����������ݣ�
a����Ӧǰ����������Ʒ������m1g b����Ӧ�������й��������Ϊm2g
c��װ��Cʵ�������m3g d��װ��Dʵ�������m4g
ijͬѧѡ��b��d��c��d����������x��y��ֵ������װ��E����ʵ��ⶨ����� ���ƫ����ƫС������Ӱ�족��������Ϊ����ѡ�������� ��ѡ����ţ������������Ҳ�����x��y��ֵ��
��7��CaSO4?2H2O���Ȼ���ʧȥ�ᾧˮ��ȡ����CaSO4?2H2O����3.44g�����ڣ�5����ʵ��װ��B�н��м��ȣ��ⶨ�����������¶ȵı仯�����ͼ��ʾ����G�����Ļ�ѧʽ�� ��

�ڽ�T2��1400���¶ȶμ��ȹ���������������ͨ������KMnO4��Һ�У���Һ��ɫ����H��I�η�����Ӧ�Ļ�ѧ����ʽΪ ��

