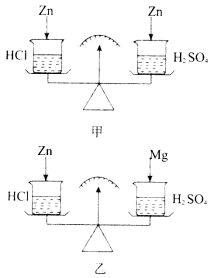
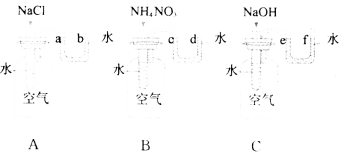
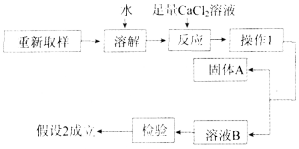
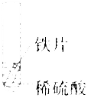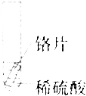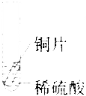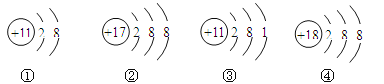ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΘ®7Ζ÷Θ©ΈΣ≤βΕ®Ρ≥¥ΩΦν―υΤΖΘ®Κ§«β―θΜ·ΡΤ‘”÷ Θ©÷–ΧΦΥαΡΤΒΡΚ§ΝΩΘ§Ϋχ––»γœ¬ Β―ιΘΚ»Γ65g¥ΩΦν―υΤΖΤΫΨυΖ÷ΈΣ5ΖίΘ§Ζ÷±πΦ”»κœύΆ§÷ ΝΩΖ÷ ΐΒΡœΓ―ΈΥα»ή“ΚΘ§ΜώΒΟ»γœ¬ Β―ι ΐΨίΘΚ
Β―ι | 1 | 2 | 3 | 4 | 5 |
―υΤΖ÷ ΝΩ/g | 13 | 13 | 13 | 13 | 13 |
Φ”»κ―ΈΥα÷ ΝΩ/g | 15 | 30 | 80 | 130 | 150 |
Ζ¥”ΠΚσΈο÷ ÷ ΝΩ/g | 28 | 43 | 90Θ°8 | 138Θ°6 | 158Θ°6 |
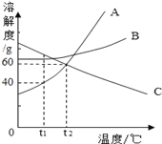
Θ®1Θ©13g―υΤΖΆξ»ΪΖ¥”Π ±Ζ≈≥ωΤχΧεΒΡ÷ ΝΩ « gΓΘ
Θ®2Θ©¥ΩΦν―υΤΖ÷–Na2CO3ΒΡ÷ ΝΩΖ÷ ΐ «Εύ…ΌΘΩΘ®–¥≥ωΦΤΥψΙΐ≥ΧΘ§ΫαΙϊ±ΘΝτ–Γ ΐΒψΚσ“ΜΈΜΘ©
Θ®3Θ©13g―υΤΖ”κ―ΈΥα«ΓΚΟΆξ»ΪΖ¥”ΠΚσ…ζ≥…Έο»ή“Κ÷–»ή÷ ΒΡ÷ ΝΩΖ÷ ΐ « Θ®÷Μ–¥ΫαΙϊΘ§±ΘΝτ–Γ ΐΒψΚσ“ΜΈΜΘ©ΓΘ
Θ®4Θ©»τœρ13g―υΤΖ÷–≥÷–χ≤ΜΕœΒΡΦ”»κœΓ―ΈΥαΘ§«κΡψΜ≠≥ωΦ”»κœΓ―ΈΥα”κ…ζ≥…ΤχΧεΒΡ÷ ΝΩΙΊœΒΆΦΓΘΘ®‘Ύ¥πΧβΩ®ΒΡΉχ±ξ÷–ΉςΆΦΘ©
ΓΨ¥πΑΗΓΩΘ®1Θ©4Θ°4 Θ®2Θ©81Θ°5% Θ®3Θ©11Θ°0%
ΓΨΫβΈωΓΩ
‘ΧβΖ÷ΈωΘΚ
Θ®1Θ©ΗυΨί÷ ΝΩ ΊΚψΕ®¬…Ω…÷Σ»ή“Κ÷ ΝΩΒΡΦθ…ΌΨΆ «…ζ≥…ΒΡΕΰ―θΜ·ΧΦΒΡ÷ ΝΩΘΚ143g-138Θ°6g=4Θ°4gΘΜ
Θ®2Θ©άϊ”ΟΜ·―ßΖΫ≥Χ ΫΘ§ΗυΨίΖ¥”ΠΒΡ÷ ΝΩ±»Φ¥Ω…ΦΤΥψ≥ω―υΤΖ÷–ΧΦΥαΡΤΒΡ÷ ΝΩΘΜ
…η¥ΩΦν―υΤΖ÷–ΧΦΥαΡΤΒΡ÷ ΝΩΈΣxΘ§¬»Μ·«βΒΡ÷ ΝΩΈΣyΘΜ
Na2CO3+2HCl==2NaCl+H2O+CO2Γϋ
106 73 44
x y 4Θ°4g
106:44=x:4Θ°4g 73ΘΚ44=Y:4Θ°4g
X=10Θ°6g y=7Θ°3g
ΧΦΥαΡΤΒΡ÷ ΝΩΖ÷ ΐΈΣΘΚ 10Θ°6gΘ·13g ΓΝ100%=81Θ°5% ―ΈΥαΒΡ÷ ΝΩΖ÷ ΐ=7Θ°3gΘ·100g ΓΝ100%=7Θ°3%
―ΈΥα÷–¬»Μ·«βΒΡ÷ ΝΩΈΣΘΚ130gΓΝ7Θ°3%=9Θ°49gΘΜΗυΨίHCl~~~~NaCl,…ζ≥…ΒΡ¬»Μ·ΡΤΒΡ÷ ΝΩΈΣz
36Θ°5 58Θ°5
9Θ°49g Z
36Θ°5Θ·58Θ°5=9Θ°49gΘ·z
Z=15Θ°21g
»ή“ΚΒΡ÷ ΝΩΈΣΘΚ130g+13g-4Θ°4g=138Θ°6gΘ§Ι »ή“ΚΒΡ»ή÷ ÷ ΝΩΖ÷ ΐΈΣ15Θ°21gΘ·138Θ°6g ΓΝ100%=11Θ°0%
Θ®4Θ©»τœρ13g―υΤΖ÷–≥÷–χ≤ΜΕœΒΡΦ”»κœΓ―ΈΥαΘ§«κΡψΜ≠≥ωΦ”»κœΓ―ΈΥα”κ…ζ≥…ΤχΧεΒΡ÷ ΝΩΙΊœΒΆΦΈΣΘΚ
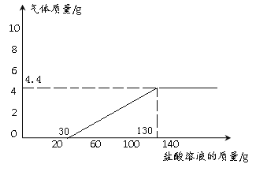

 “ΜΩΈ“ΜΝΖΩΈ ±¥ο±ξœΒΝ–¥πΑΗ
“ΜΩΈ“ΜΝΖΩΈ ±¥ο±ξœΒΝ–¥πΑΗ