��Ŀ����
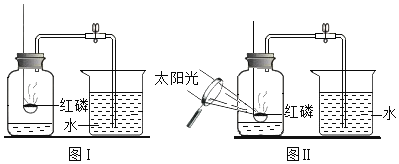
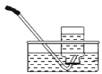
����Ŀ������и�ǿ�ȡ����۵㡢����ʴ���ŵ㣬�ڹ�ҵ�ϵõ��㷺�����á�ͼ1�ǻ����������Ʊ�������IJ�������ͼ���������ʲ��������Ӧ����
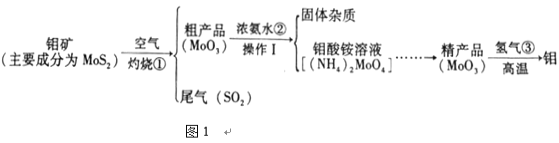
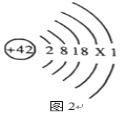
��1���������弰��ֲ������_______________��ѡ������Ԫ������������Ԫ��������ԭ�ӵĽṹʾ��ͼ2������X��_________________��
��2������ٷ�Ӧ�Ļ�ѧ����ʽ_________________________________��
��3���������ʿ�����������SO2β������_________������ĸ����
A ����������Һ B Ũ���� C ��ˮ
��4��(NH4)2MoO4��Mo�Ļ��ϼ���_____________����ũҵ�ϼ����������_______�ʡ�
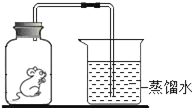
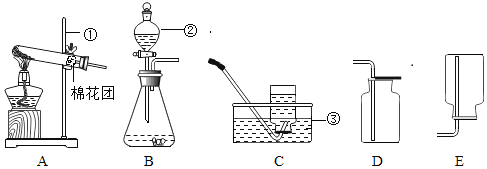
��5�������ʵ����ģ�����������Ҫʹ�õ���Ҫ����������©������������________��
��6������H2��CO�ֱ�ԭ�������ľ���Ʒ��MoO3��������Ļ�ԭ����������Ϊ____��
���𰸡���Ԫ�� 13 2Mo+7O2![]() 2MoO3+4SO2 AC +6 �� �ձ� 1��14
2MoO3+4SO2 AC +6 �� �ձ� 1��14
��������
��1���������弰��ֲ��������Ԫ�أ���ԭ���У��˵�����������������ԭ�ӵĽṹʾ��ͼ2������X��42-2-8-18-1=13��
��2����������ͼ��֪������ٷ�Ӧ��MoS2��O2�ڸ��������·�Ӧ����SO2��MoO3����Ӧ�Ļ�ѧ����ʽΪ��2Mo+7O2![]() 2MoO3+4SO2��
2MoO3+4SO2��
��3���������������������ơ���ˮ��Ӧ��������Ũ���ᷴӦ������������SO2β����������������Һ�Ͱ�ˮ����ѡAC��
��4����(NH4)2MoO4�У�笠��ӡ���Ԫ�صĻ��ϼ۷ֱ�Ϊ+1��-2���裺(NH4)2MoO4����Ļ��ϼ�Ϊx�����ݻ�������Ԫ�صĻ��ϼ۵Ĵ�����Ϊ�㣬���У���+1����2+x+��-2����4=0
���x=+6��(NH4)2MoO4��Mo�Ļ��ϼ���+6��(NH4)2MoO4�к��е�Ԫ�أ���ũҵ�ϼ���������ǵ��ʣ�
��5���������ǽ�������Һ����롣�����ʵ����ģ�����������Ҫʹ�õ���Ҫ����������©�������������ձ���
��6������H2��CO�ֱ�ԭ�������ľ���Ʒ��MoO3��������Ļ�ԭ���ֱ�ΪH2��CO��
�裺MoO3������Ϊm������Ļ�ԭ���ֱ�ΪH2��CO�����ֱ�Ϊx��y��
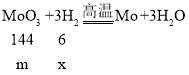
![]()
![]() ��
��
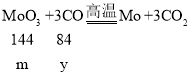
![]()
![]() ��
��![]() ������H2��CO�ֱ�ԭ�������ľ���Ʒ��MoO3��������Ļ�ԭ����������Ϊ1:14��
������H2��CO�ֱ�ԭ�������ľ���Ʒ��MoO3��������Ļ�ԭ����������Ϊ1:14��

 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�����Ŀ�����������ǻ�ѧ�г�����һ��ҩƷ������dz�ư�ɫ������ĩ���ڿ���������ˮ�ֺͶ�����̼��������ˮ����ˮ���ܷ�����Ӧ�����������ƺ������⣬����ʽ��Na2O2+2H2O=2NaOH+H2O2�������������ֽܷ�ų�������
(1)����ˮ���������ʵ�鷽���ǣ�_____�������ķ�Ӧ����ʽ�ǣ�_____��
(2)ijͬѧΪ��֤���������Ƽ���ˮ���������������ƣ������ɵ���Һ�еμ��˼�����ɫ��̪��Һ���۲쵽����������Һ�ȱ�����Ϊ��ɫ������Դ��������̽����
��������⣩��ʲô������Һ�ȱ������ɫ��
����������裩������1����ͬѧ����������ɫ��̪��Һ���ʵ�����Һ��ɫ����
����2����ͬѧ�����������ɵĹ�������ʹ��Һ��ɫ����
��ʵ������ۣ���a����ͬѧ����IJ��룬���������ͬѧ�ķ��ԣ���Ϊ_____��
b��Ϊ����֤�Լ��IJ��룬��ͬѧ��������·�����ʵ����֤��
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ����ϡ����������Һ���Թ��У��μ�1��2�η�̪��Һ������_____�۲����� | ��Һ��ȻΪ_____ɫ | _____ |
�����۽��������㻹�������ͬ�IJ�����
����3��_____��
����3��
ʵ�鲽�� | ʵ������ | ʵ����� |
_____ | _____ | _____ |