��Ŀ����
��ͭ����ͭ��п����ɵĺϽ���;�㷺��ij��ȤС��Ϊ̽����ͭ�Ͻ����ɣ�ȡ20.00g��ĩ״��ͭ�Ͻ���Ʒ����60.00gϡ����ƽ���ֳ����ȷݣ������μ�����Ʒ�У����������������ַ�Ӧ���˳����壬����ϴ�ӡ������������ʵ���������£�
| ��һ�� | �ڶ��� | ������ | |
| ʣ����������/g | 16.75 | 13.50 | 12.40 |
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
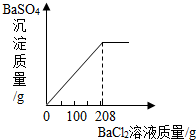
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ��ͼ��ʾ��������BaCl2��Һ����������������

�⣺��1����Ϊͭ��п����ɻƽ��У�ͭ����ϡ���ᷴӦ�������ɱ����֪ͭ������Ϊ12.40g��
��п������=20.00-12.40=7.60g��пԪ�ص���������= ��100%=
��100%= ��100%=38%
��100%=38%
��2���ɱ����֪��һ��ʣ�����16.75g���ڶ���Ϊ13.50g��˵���ڶ��η�Ӧ��п������Ϊ16.75-13.50=3.25g��
��ڶ���ʵ���зų����������ΪX����������ã�
Zn+H2SO4=ZnSO4+H2��
65 2
3.25g X
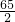 =
=
��ã�X=0.1g
��3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ����BaSO4������ʵ������������60.00gϡ���ᷴӦ���ã��ɣ�2�������20.00������μӷ�Ӧ������Ϊ Y��
 =
= ��ã�Y=4.9g
��ã�Y=4.9g
20.00����������ʵ���������=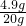 ��100%=24.5%
��100%=24.5%
��ô60.00�����������ʵ�����=60.00g��24.5%=14.7g
������BaCl2��Һ����������ΪZ��
�ֹ�ϵ H2SO4��BaCl2
98 208
14.7g Z
 =
= ��Z=31.2g��
��Z=31.2g��
BaCl2��Һ��������������= ��100%=15%
��100%=15%
�𣺣�1���û�ͭ�Ͻ��У�пԪ�ص���������38%��
��2���ڶ���ʵ���зų����������0.1g��
��3��BaCl2��Һ��������������15%��
��������1��Ҫ��п��������������֪�Ͻ����������ͭ�����ᷴӦ�ӱ����֪�������ͭ������Ϊ12.40�ˣ���ôп���������Լ����������пԪ�ص���������= ��100%����ã���2��Ҫ��ڶ���ʵ���зų�������������ӱ����п�֪��һ�κ͵ڶ���֮��������˵��������Ǽ��ٵ�п�����ᷴӦ���ɵ������ˣ��ɴ˸��ݻ�ѧ����ʽ��֪��Ӧ�����������������������3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ���ɲ�����ˮ��BaSO4��ɫ������ʵ�����Ǻ�ϡ�����е���������ӽ�ϣ�����ֻҪ���������������Ϳ�������Ȼ�����������
��100%����ã���2��Ҫ��ڶ���ʵ���зų�������������ӱ����п�֪��һ�κ͵ڶ���֮��������˵��������Ǽ��ٵ�п�����ᷴӦ���ɵ������ˣ��ɴ˸��ݻ�ѧ����ʽ��֪��Ӧ�����������������������3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ���ɲ�����ˮ��BaSO4��ɫ������ʵ�����Ǻ�ϡ�����е���������ӽ�ϣ�����ֻҪ���������������Ϳ�������Ȼ�����������
���������⿼����ͼʾ�ͻ�ѧ��Ӧ���ϵ�ͨ����ѧ����ʽ���м��㣬�����Ѷ��ϴ����Ĺؼ����ܹ��ӱ������ҳ�������ϵ�����շ�Ӧ���������ѧ����ͼ������ͼʾ������Ҫ��ϸߣ�
��п������=20.00-12.40=7.60g��пԪ�ص���������=
 ��100%=
��100%= ��100%=38%
��100%=38%��2���ɱ����֪��һ��ʣ�����16.75g���ڶ���Ϊ13.50g��˵���ڶ��η�Ӧ��п������Ϊ16.75-13.50=3.25g��
��ڶ���ʵ���зų����������ΪX����������ã�
Zn+H2SO4=ZnSO4+H2��
65 2
3.25g X
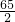 =
=
��ã�X=0.1g
��3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ����BaSO4������ʵ������������60.00gϡ���ᷴӦ���ã��ɣ�2�������20.00������μӷ�Ӧ������Ϊ Y��
 =
= ��ã�Y=4.9g
��ã�Y=4.9g 20.00����������ʵ���������=
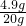 ��100%=24.5%
��100%=24.5%��ô60.00�����������ʵ�����=60.00g��24.5%=14.7g
������BaCl2��Һ����������ΪZ��
�ֹ�ϵ H2SO4��BaCl2
98 208
14.7g Z
 =
= ��Z=31.2g��
��Z=31.2g��BaCl2��Һ��������������=
 ��100%=15%
��100%=15%�𣺣�1���û�ͭ�Ͻ��У�пԪ�ص���������38%��
��2���ڶ���ʵ���зų����������0.1g��
��3��BaCl2��Һ��������������15%��
��������1��Ҫ��п��������������֪�Ͻ����������ͭ�����ᷴӦ�ӱ����֪�������ͭ������Ϊ12.40�ˣ���ôп���������Լ����������пԪ�ص���������=
 ��100%����ã���2��Ҫ��ڶ���ʵ���зų�������������ӱ����п�֪��һ�κ͵ڶ���֮��������˵��������Ǽ��ٵ�п�����ᷴӦ���ɵ������ˣ��ɴ˸��ݻ�ѧ����ʽ��֪��Ӧ�����������������������3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ���ɲ�����ˮ��BaSO4��ɫ������ʵ�����Ǻ�ϡ�����е���������ӽ�ϣ�����ֻҪ���������������Ϳ�������Ȼ�����������
��100%����ã���2��Ҫ��ڶ���ʵ���зų�������������ӱ����п�֪��һ�κ͵ڶ���֮��������˵��������Ǽ��ٵ�п�����ᷴӦ���ɵ������ˣ��ɴ˸��ݻ�ѧ����ʽ��֪��Ӧ�����������������������3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ���ɲ�����ˮ��BaSO4��ɫ������ʵ�����Ǻ�ϡ�����е���������ӽ�ϣ�����ֻҪ���������������Ϳ�������Ȼ��������������������⿼����ͼʾ�ͻ�ѧ��Ӧ���ϵ�ͨ����ѧ����ʽ���м��㣬�����Ѷ��ϴ����Ĺؼ����ܹ��ӱ������ҳ�������ϵ�����շ�Ӧ���������ѧ����ͼ������ͼʾ������Ҫ��ϸߣ�

��ϰ��ϵ�д�
�����Ŀ
��ͭ����ͭ��п����ɵĺϽ���;�㷺��ij��ȤС��Ϊ̽����ͭ�Ͻ����ɣ�ȡ20.00g��ĩ״��ͭ�Ͻ���Ʒ����60.00gϡ����ƽ���ֳ����ȷݣ������μ�����Ʒ�У����������������ַ�Ӧ���˳����壬����ϴ�ӡ������������ʵ���������£�
����㣺��Ҫ��д��������̣�
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
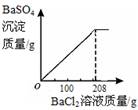
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ��ͼ��ʾ��������BaCl2��Һ����������������
| ��һ�� | �ڶ��� | ������ | |
| ʣ����������/g | 16.75 | 13.50 | 12.40 |
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
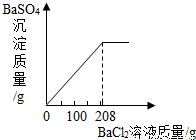
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ��ͼ��ʾ��������BaCl2��Һ����������������

��3�֣���ͭ����ͭ��п����ɵĺϽ���;�㷺��ij��ȤС��Ϊ̽����ͭ�Ͻ����ɣ�ȡ20.00 g��ĩ״��ͭ�Ͻ���Ʒ����60.00 gϡ����ƽ���ֳ����ȷݣ������μ�����Ʒ�У����������������ַ�Ӧ���˳����壬����ϴ�ӡ������������ʵ���������£�
|
|
��һ�� |
�ڶ��� |
������ |
|
ʣ������������g |
16.75 |
13.50 |
12.40 |
����㣺��Ҫ��д��������̣�
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ����ͼ��ʾ��������BaCl2��Һ����������������
��2010?��������ģ����ͭ����ͭ��п����ɵĺϽ���;�㷺��ij��ȤС��Ϊ̽����ͭ�Ͻ����ɣ�ȡ20.00g��ĩ״��ͭ�Ͻ���Ʒ����60.00gϡ����ƽ���ֳ����ȷݣ������μ�����Ʒ�У����������������ַ�Ӧ���˳����壬����ϴ�ӡ������������ʵ���������£�
����㣺��Ҫ��д��������̣�
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ��ͼ��ʾ��������BaCl2��Һ����������������
| ��һ�� | �ڶ��� | ������ | |
| ʣ����������/g | 16.75 | 13.50 | 12.40 |
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ��ͼ��ʾ��������BaCl2��Һ����������������


