��Ŀ����
��7�֣�����������������������Ҫ���á�
��1��ͭ���������ߣ���Ҫ�����˽���ͭ����չ�Ժ� ��
��2��������(��Ҫ�ɷ�ΪFe2O3)��һ����̼�����Ļ�ѧ����ʽΪ ��
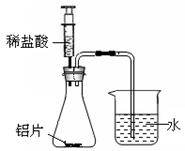
��3��Ϊ�˷�ֹ����ˮ��ͷ���⣬����泣��һ������ô�ʩ�����ԭ����_____________��
��4����Ag��Fe��Cu���ֽ������˳���̽����������ѡ�Լ��鲻���е��� ��
��Fe��Ag��CuSO4��Һ ��Cu��Ag��FeSO4��Һ ��Cu��FeSO4��Һ��AgNO3��Һ
��5����ѧС����ʵ��������������·�Һ������
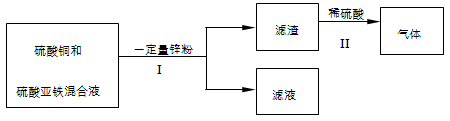
�٢��еIJ��������� ��
��д������һ��������Ӧ�Ļ�ѧ����ʽ ��
����Һ����ɿ����� ��
��1��ͭ���������ߣ���Ҫ�����˽���ͭ����չ�Ժ� ��
��2��������(��Ҫ�ɷ�ΪFe2O3)��һ����̼�����Ļ�ѧ����ʽΪ ��
��3��Ϊ�˷�ֹ����ˮ��ͷ���⣬����泣��һ������ô�ʩ�����ԭ����_____________��
��4����Ag��Fe��Cu���ֽ������˳���̽����������ѡ�Լ��鲻���е��� ��
��Fe��Ag��CuSO4��Һ ��Cu��Ag��FeSO4��Һ ��Cu��FeSO4��Һ��AgNO3��Һ
��5����ѧС����ʵ��������������·�Һ������

�٢��еIJ��������� ��
��д������һ��������Ӧ�Ļ�ѧ����ʽ ��
����Һ����ɿ����� ��
��1�������� ��2��Fe2O3 + 3CO  2Fe + 3CO2
2Fe + 3CO2
��3������������ˮ ��4����
��5���ٹ��� ��Fe + H2SO4 ="==" Fe SO4 + H2��
��������������������
 2Fe + 3CO2
2Fe + 3CO2��3������������ˮ ��4����
��5���ٹ��� ��Fe + H2SO4 ="==" Fe SO4 + H2��
��������������������
���������(1)���ʾ�����;����;��ӳ���ʣ�ͭ���������ߣ���Ҫ�����˽���ͭ����չ�Ժ͵�����
(2) ������(��Ҫ�ɷ�ΪFe2O3)��һ����̼�����Ļ�ѧ����ʽΪ��Fe2O3 + 3CO
 2Fe + 3CO2
2Fe + 3CO2(3) ���������������������ˮ�Ӵ�������Ϊ�˷�ֹ����ˮ��ͷ���⣬����泣��һ������ô�ʩ�����ԭ���ǣ�����������ˮ
(4)̽�����ֽ����Ļ�ԣ������õ�ԭ����1���������ᷴӦ�������Ƿ�Ӧ��Ӧ�ļ��ҳ̶����жϻ�Ե�ǿ����2������������Һ��Ӧ��һ��������ֽ�����һ������Һ��Ӧ��һ�ֽ����Ͷ�������Һ��Ӧ������Ag��Fe��Cu���ֽ������˳���̽������Cu��Ag��FeSO4��Һ�����У�ֻ��֤�����Ļ����ǿ��Cu��Ag�Ļ��ǿ���Ƚϲ��ˣ���ѡ��
(5) �ٽ��в�������ǽ������Һ����룬�ǹ���
��ʵ������ϡ����������ݣ�˵��������һ�������������Ԣ���һ��������Ӧ�Ļ�ѧ����ʽ��Fe + H2SO4 ="==" Fe SO4 + H2��
�۸��ݢڿ�֪������һ������п�ۣ�һ������������2����Ӧ��CuSO4+Zn==ZnSO4+Cu��FeSO4+Zn==ZnSO4+Fe����������������˵����һ����Ӧ�Ѿ���ȫ��Ӧ���ڶ�����ӦҲһ�����������ڷ����ij̶ȾͲ�ȷ����������Һ����ɿ���������п������п����������

��ϰ��ϵ�д�
�����Ŀ