��Ŀ����
ʯ�ҳ�Ϊ�˲ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ��4 gʯ��ʯ��Ʒ����20 gϡ�����4�μ�����Ʒ��(��Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦ��Ҳ������ˮ)����ַ�Ӧ���ˡ�����Ȳ���������������ʵ���������±���
| ϡ��������� | ��һ�μ���5 g | �ڶ��μ���5 g | �����μ���5 g | ���Ĵμ���5 g |
| ʣ���������� | 3 g | 2 g | l g | 1 g |
(1) �����ʯ��ʯ��Ʒ��̼��Ƶ�����������
(2)�����20 gϡ�����к���HCI������������ȷ��0.01��
(1) (3��) �⣺��ʯ��ʯ��Ʒ��̼��Ƶ��������� �� ![]() ��75��
��75��
(2)(6��)��1���ɱ������ݿ�֪�����μ���5 gϡ�����3 g̼�����15 gϡ����պ���ȫ��Ӧ��
��15 gϡ�����е�HCl����Ϊx
CaC03 + 2HCl === CaCl2 + H20 + C02 ��----------(2��)
100 73
3g x
![]() =
= ![]() ----------------------------------(2��)
----------------------------------(2��)
x=2.19 g ----------------------------------(1��)
20 gϡ���������HCI����Ϊ��2.19 g��20 g��15 g =2.92 g ----------(1��)
�𣺣��ԣ�
��2���ɱ������ݿ�֪��һ�Σ��ڶ��Σ�����5 gϡ�����1 g̼�����5 gϡ����պ���ȫ��Ӧ��
��5 gϡ�����е�HCl����Ϊx
CaC03 + 2HCl === CaCl2 + H20 + C02 ��----------(2��)
100 73
1g x
![]() =
=![]() ----------------------------------(2��)
----------------------------------(2��)
x=0.73 g ---------------------------------- (1��)
20 gϡ���������HCI����Ϊ��0.73 g��4 =2.92 g ------------- (1��)
�𣺣��ԣ�

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�| ϡ��������� | ��һ�μ���5g | �ڶ��μ���5g | �����μ���5g | ���Ĵμ���5g |
| ʣ���������� | 3g | 2g | 1g | 1g |
��2�������20gϡ�����к���HCl������������ȷ��0.01��
| ϡ��������� | ��һ�μ���5g | �ڶ��μ���5g | �����μ���5g | ���Ĵμ���5g |
| ʣ���������� | 3g | 2g | 1g | 1g |
��2�������ϡ�������������������д��������̣������ȷ��0.1%����
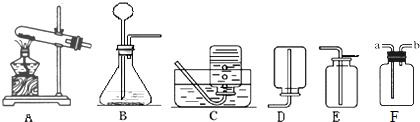
��1��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼��Ӧѡ�õ�װ����
��2��ʵ������Aװ�ü��ȸ����������ȡ��������Ҫ�Ľ�����
�ٹ���������Һ�Ͷ��������������� ��һ����̼��ԭ�������� ��ľ̿��ԭ����ͭ
��3��װ��F�������ռ����壬������������;������Fƿ��װ��
��4��ʯ�ҳ�Ϊ�˲ⶨһ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ��4gʯ��ʯ��Ʒ����20gϡ�����4�μ�����Ʒ�У���Ʒ�г�̼����⣬����ijɷּȲ������ᷴӦ��Ҳ������ˮ������ַ�Ӧ���ˡ�����Ȳ���������������ʵ���������±���
| ϡ��������� | ��һ�μ���5g | �ڶ��μ���5g | �����μ���5g | ���Ĵμ���5g |
| ʣ���������� | 3g | 2g | l g | 1g |