��Ŀ����
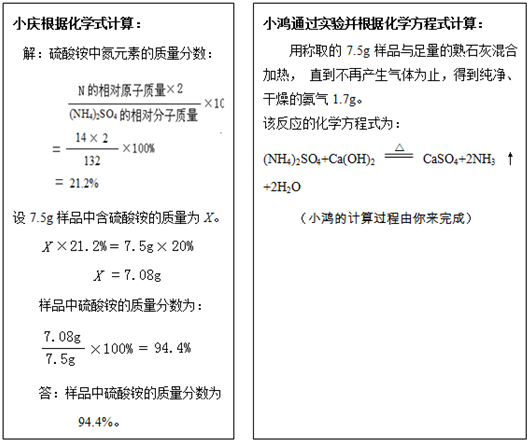
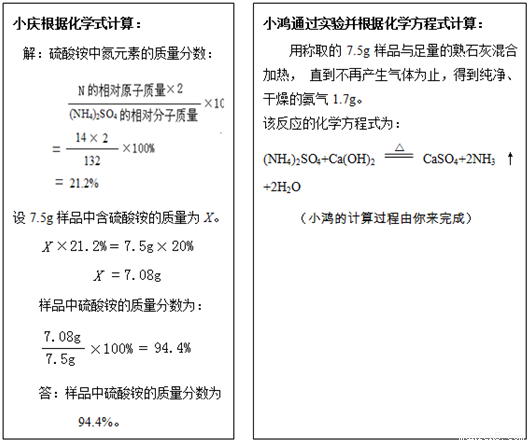
����۵���Ҫ�ɷ�������李�����һ�����к�����Ϊ20.0%�ķ������Ʒ��С���С��Ϊ�˼���÷����������淋��������������dz�ȡ7 .5g��Ʒ���ֱ�������з�����
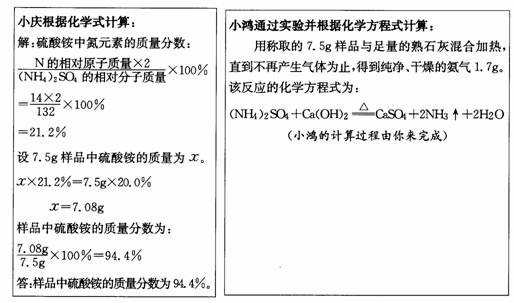
��1���������С���ʵ������������Ʒ�����ᰴ����������������������ȷ��0 .1 % )
��2����ļ�������С��ļ�����__________ �����ͬ������ͬ��' ) ,
ԭ����_______________________________________________ ��
��3������С��ʵ��ķ�Ӧԭ��������ʩ�÷����ʱӦע��___________________��
�������õ������ԭ��������H-1 C-12 N-14 O-16 S-32 ��
��1���⣺��7.5g��Ʒ�к�������淋�����Ϊ![]() ��
��
��NH4��2SO4+Ca(OH)2![]() CaSO4+2NH3��+2H2O
CaSO4+2NH3��+2H2O
132 34
![]() 1.7g
1.7g
![]()
![]() =
=![]()
=6.6g
��Ʒ�����������������
![]()
����Ʒ�к����������������Ϊ88.8%��
��2������ͬ��С�����ռ������а�����ɢ������Ʒ�к�����С��20.0%���������𰸾��ɣ���
��3��������������ʻ��ʩ�á�
 |

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ