��Ŀ����
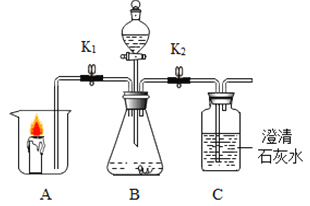
����Ŀ������ͼ��ʾװ�òⶨ�����������ĺ�����ʵ��ʱ�����ݻ�Ϊ450mL�ļ���ƿ��װ��150mL���к�īˮ��50��ˮ���ڴ���ȼ�ճ��з��������ף���Ͳ��ʢ������50��ˮ����ͼ����������ˮ�²��������£���Ӧ����Ͳ��˭������Ϸ�Ӧǰ������60mL
��1��ȼ�ճ�����ˮ�棬������ȼ�գ�˵��ȼ����Ҫʲô������_________������ȼ�ջ�ѧ����ʽΪ______________��
��2��ʵ������������Ͳ��ˮ����ԭ����________________��
��3������ʵ����������������������������ԼΪ____________��д������ʽ�ͽ������
��4������ƿ�еIJ�������Ϊʲô����Һ�����£���д�����ɣ�_______________��
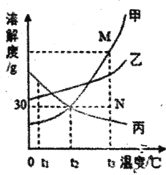
��5����ͼһ��ijͬѧ�����������������ⶨ�ܱ���������������ȼ��ʱ����ƿ�ڴ������ĺ����仯���õ�ͼ������ͼ������ݸ�����ͼ�ش��������⡣
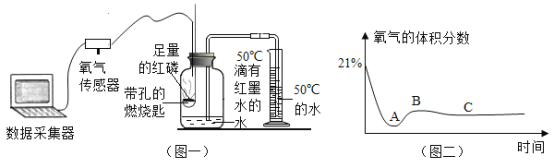
��������A��B��C�����У������Ǵ�_________�㿪ʼϨ��ġ�
������۽ǶȽ���BC�����������½��������ȶ���ԭ��____________��
�۸�������ͼ��Ϣ�����ȼ����ʲô�µ���ʶ________________��
���𰸡���ȼ����������������Ӵ� 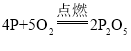 ����ȼ�շ��ȣ�ʹ����ƿ��ѹǿ�������к�īˮ��ˮѹ����Ͳ�� 60(450-150)=20�� Һ�⣬��ֹȼ�շ��ȣ�ʹװ���ڿ��������ݳ����Բ���������Ӱ�� A ���Ӳ�ͣ�˶������������Ͼ��� ��ȼ����Ҫ��һ��Ũ�ȵ������в���ȼ��
����ȼ�շ��ȣ�ʹ����ƿ��ѹǿ�������к�īˮ��ˮѹ����Ͳ�� 60(450-150)=20�� Һ�⣬��ֹȼ�շ��ȣ�ʹװ���ڿ��������ݳ����Բ���������Ӱ�� A ���Ӳ�ͣ�˶������������Ͼ��� ��ȼ����Ҫ��һ��Ũ�ȵ������в���ȼ��
��������
��1��ȼ�ճ�����ˮ�棬������ȼ�գ�˵��ȼ�ձ����������Ӵ�������ȼ���������������ף���Ӧ�Ļ�ѧ����ʽΪ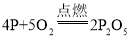 ��
��
��2������ȼ�շų�����������ƿ��ѹǿ���Ὣ���к�īˮ��ˮѹ����Ͳ�У�
��3������ʵ�������������������������=![]() ��
��
��4������ƿ�еIJ������ܲ���Һ�����£���ֹȼ�շ��ȣ�����ƿ��ѹǿ����ʹװ���ڿ��������ݳ����Բ���������Ӱ�죻
��5���ٰ���ȼ����Ҫ�������������ľ�ʱȼ�յİ���Ϩ�𣬷���ͼ��֪�Ǵ�A�㿪ʼϨ��ģ�
������ƿ�ڵ��¶��ָ������£������˶����������������ܻ�Ͼ��ȣ�ƿ��ѹǿ���ٱ仯��
�۸�������ͼ��Ϣ��֪����ȼ����Ҫ��һ��Ũ�ȵ������в���ȼ�ա�

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ��һ�������£���һ���ܱ����������������ʣ���ַ�Ӧ��÷�Ӧǰ������ʵ��������£���������������ȷ����
���� | A | O2 | CO2 | H2O | N2 |
��Ӧǰ����/g | 24.4 | 109.6 | 2.4 | 1.2 | 5.6 |
��Ӧ������/g | 0 | 100 | 20 | 12 | X |
A.����A��̼Ԫ�غ���Ԫ�ص�������Ϊ4��1
B.����A��̼����͵�����Ԫ�����
C.x=11.2
D.������������������������������
����Ŀ�����ʵ���ɡ��ṹ���������Ʊ�
��1�����ʵ���ɺͽṹ���������ʵ����ʡ������������еĻ�ѧ֪ʶ����±������
���ʵ����ƣ����׳ƣ� | �������ʵ������� | �������ʻ�ѧ���ʵı仯���÷���ʽ��ʾ��дһ�����ɣ� |
_________ | Na+��OH- | _________ |
�Ҵ� | _________ | _________ |
ͭ | _________ | _________ |
�ϱ����оٵ��������ʻ�ѧ���ʵķ�Ӧ�У������Ļ�����Ӧ������______��
��2���������ʵ����ʿ��Լ����������ʡ�ʵ������һƿʧȥ�������Һ��Ϊ�˼�������ϡ���ỹ���Ȼ�þ��Һ��С��ͬѧѡ�ò�ͬ���Լ�����ʵ�飬����������˳�����
�ɹ�ѡ����Լ�����Ƭ���������������״ʯ��ʯ��������̼����������Һ�������ʯ��ˮ
�밴Ҫ����д�±���ֻ��д��������пɹ۲쵽��������Ļ�ѧ�仯����ʽ��
��������з�����ѧ�仯�ķ���ʽ | �������ʵ����� |
_________ | ������ijЩ�Ӧ |
_________ | _________ |
_________ | _________ |
��3���������ʵ����ʿ�ͨ����ѧ�仯�Ʊ����ʵ�����CuSO4��5H2O���ڹ�ũҵ�������й㷺����;��ʵ�������÷Ͼɵ�ص�ͭ������Ҫ��ͭ��п��Ϊԭ���Ʊ�������ʵ��������ͼ��ʾ���������ϣ�����п����������������Һ��
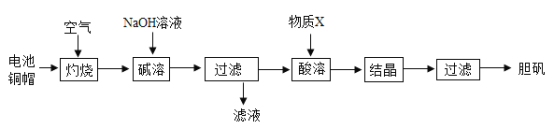
�ٷϾɵ�ظ�ñ�ı��泣�����ۣ�����ϴ�ྫԤ�ȳ�ȥ��ϴ�ྫ�ڳ�ȥ���۵Ĺ�������____________���á�
���������������У�Ϊ�ӿ췴Ӧ���ʿɲ�ȡ���ִ�ʩ������衢______��______�ȡ�
����������ʱ��������Ӧ�Ļ�ѧ����ʽ��_____________��
����д�������ڹ�ũҵ�����е�һ����;_______________.