��Ŀ����
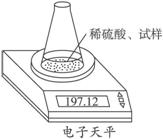
 ��ͼʾװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00 g����ƿ����140.00 g����������ϡ���ᣨ����Ϊ50.00 g��ÿ����ͬʱ�����һ�Σ��������±���
��ͼʾװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00 g����ƿ����140.00 g����������ϡ���ᣨ����Ϊ50.00 g��ÿ����ͬʱ�����һ�Σ��������±���| �������� | ����/g | |
| ��ƿ+����+ϡ���� | ��1�� | 197.64 |
| ��2�� | 197.48 | |
| ��3�� | 197.26 | |
| ��4�� | 197.16 | |
| ��5�� | 197.12 | |
| ��6�� | 197.12 |
��2�������������Ƶ�����������
��3������ϡ������H2SO4���������������ڶ��٣�
�ʴ�Ϊ����5�κ͵�6�ζ�����ͬ���ѳ�ַ�Ӧ��
��2��CO2������m��CO2��=��8.00 g+140.00 g+50.00 g��-197.12 g=0.88 g
��������Ʒ��Na2CO3������Ϊx��
Na2CO3+H2SO4�TH2O+CO2��+Na2SO4
106 44
x 0.88 g
106��44=x��0.88g ��֮�� x=2.12 g
����ȡ��Ʒ��NaOH����������=
| 8.00g-2.12g |
| 8.00g |
��3������Ʒ��Na2CO3����H2SO4������Ϊy��
Na2CO3+H2SO4�TH2O+CO2��+Na2SO4
106 98
2.12 g y
106��98=2.12g��y ��֮�� y=1.96 g
������NaOH������=8.00g-2.12g=5.88g����������NaOH����H2SO4������Ϊz��
2NaOH+H2SO4�T2H2O+Na2SO4
80 98
5.88g z
80��98=5.88g��z ��֮�� z=7.20 g
��ϡ������H2SO4����������������
| 1.96g+7.20g |
| 50.00g |
�𣺣�2���������Ƶ���������Ϊ73.5%��
��3������ϡ������H2SO4����������������18.32%��

 �żӾ���ϵ�д�
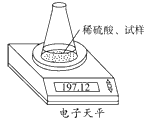
�żӾ���ϵ�д���ͼʾװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00 g����ƿ����140.00 g����������ϡ����(����Ϊ50.00 g)ÿ����ͬʱ�����һ�Σ��������±���

| �������� | ����/g | |
| ��ƿ+����+ϡ���� | ��1�� | 197.64 |
| ��2�� | 197.48 | |
| ��3�� | 197.26 | |
| ��4�� | 197.16 | |
| ��5�� | 197.12 | |
| ��6�� | 197.12 |
(1)���ؽ��е��ߴζ�����ԭ����___________________________________��
(2)�����������Ƶ�����������
(3)����ϡ������H2SO4���������������ڶ��٣�
��ͼʾװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00 g����ƿ����140.00 g����������ϡ����(����Ϊ50.00 g)ÿ����ͬʱ�����һ�Σ��������±���
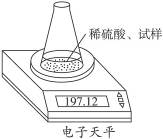
| �������� | ����/g | |
| ��ƿ+����+ϡ���� | ��1�� | 197.64 |
| ��2�� | 197.48 | |
| ��3�� | 197.26 | |
| ��4�� | 197.16 | |
| ��5�� | 197.12 | |
| ��6�� | 197.12 |
(1)���ؽ��е��ߴζ�����ԭ����___________________________________��
(2)�����������Ƶ�����������
(3)����ϡ������H2SO4���������������ڶ��٣�
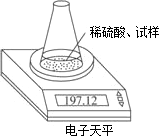
��ͼʾװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00 g����ƿ����140.00 g����������ϡ����(����Ϊ50.00 g)ÿ����ͬʱ�����һ�Σ��������±���
| �������� | ����/g | |
| ��ƿ+����+ϡ���� | ��1�� | 197.64 |
| ��2�� | 197.48 | |
| ��3�� | 197.26 | |
| ��4�� | 197.16 | |
| ��5�� | 197.12 | |
| ��6�� | 197.12 |
(1)���ؽ��е��ߴζ�����ԭ����___________________________________��
(2)�����������Ƶ�����������
(3)����ϡ������H2SO4���������������ڶ��٣�