��Ŀ����
��ش������йؽ��������⡣
��1�����ǻ��ý�����Ϊʲôͨ������ȴ����ʴ?
��2���ұ���ij̽��ʵ��װ��ͼ��һ��ʱ����ܹ۲쵽��������������ʲ ô����? ��U��Һ���кα仯��(װ�����������ã��ҿ�ʼʱU�����˵ĺ�īˮҺ����ƽ)
��3��X��Y�����ֽ������壬��X��Y����ϡ�����У�Y�ܽⲢ���� ������X�ޱ仯����X������������Һ�У�X��������������
���ж�X��Y�������ֽ����Ļ����ǿ������˳��
�ھ���ȷ��X��д��X����������Һ��Ӧ�Ļ�ѧ����ʽ��
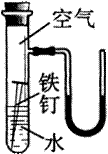
��1������������Ӧ��������������ܵ���������Ĥ�𱣻����á�
��2������������ ��U��Һ���Ϊ����ҵ͡�
��3����Y��x��Ag �� Cu+2AgNO3=Cu(NO3)2+2Ag

��ϰ��ϵ�д�
 �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
 ��2010?���ϣ���ش������йؽ��������⣮
��2010?���ϣ���ش������йؽ��������⣮
 ��ش������йؽ��������⣺
��ش������йؽ��������⣺ ��2012?Ϣ��һģ����ش������йؽ��������⣮
��2012?Ϣ��һģ����ش������йؽ��������⣮