��Ŀ����
ij�����������Ĵ����Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ�����������ij������ȤС�����������ʵ�飮ȡһ�������ĸô�����Ʒ���Թ��У�����85.6gϡ���ᣬǡ����ȫ��Ӧ������8.8g���壮���ⶨ��������Һ������Ϊ��������Һ����Һ�к���Ԫ�ص�����Ϊ10.0g������ݴ˷������㣺��1���ò�Ʒ��̼���Ƶ�����������______��
��2����Ӧ��������Һ�����ʵ�����������
���𰸡���������1���ɸ��ݶ�����̼������������̼���ƺ����ᷴӦ�Ļ�ѧ����ʽ���м��㣮
��2�����ȿ��Ը�����Һ����Ԫ�ص�����������Ԫ�������غ������Һ���Ȼ��Ƶ�������
����ٸ��ݵ�һ�������Ӧ�����Ȼ��Ƶ����������ԭ��Ʒ���Ȼ��Ƶ�������
��������Һ��������Ϊ��Ӧǰ���ʵ�������ȥ�ų�����������������������������
����⣺��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2 C03+2HCl=2NaCl+H20+C02��
106 117 44
x y 8.8g
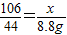 ����֮�ã�x=21.2g
����֮�ã�x=21.2g
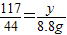 ����֮�ã�y=23.4g
����֮�ã�y=23.4g
��������Һ�����ʵ�����Ϊm��
Na--NaCl
23 58.5
10.0g m
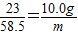 ��
��
��֮�ã�m=25.4g
��ԭ��Ʒ��NaCl������Ϊ��25.4g-23.4g=2.0g
ԭ��Ʒ������Ϊ=21.2+2.0=23.2g
������Һ������Ϊ��21.2g+2.0g+85.6g-8.8g=l00.0 g
��1����Ʒ��̼���Ƶ���������=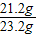 ×100%=91.4%
×100%=91.4%
��2��������Һ�����ʵ���������Ϊ��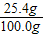 ×100%=25.4%
×100%=25.4%
�𣺣�1����Ʒ��̼���Ƶ���������91.4%��
��2��������Һ�����ʵ���������Ϊ25.4%��
������������һ�����Ѷȣ���Ҫ��ԭ��Ʒ���������������õ��˹�ϵʽ�ⷨ���ݻ�ѧ����ʽ������⣬��һ�����������⣮
��2�����ȿ��Ը�����Һ����Ԫ�ص�����������Ԫ�������غ������Һ���Ȼ��Ƶ�������
����ٸ��ݵ�һ�������Ӧ�����Ȼ��Ƶ����������ԭ��Ʒ���Ȼ��Ƶ�������
��������Һ��������Ϊ��Ӧǰ���ʵ�������ȥ�ų�����������������������������
����⣺��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2 C03+2HCl=2NaCl+H20+C02��
106 117 44
x y 8.8g
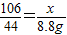 ����֮�ã�x=21.2g
����֮�ã�x=21.2g 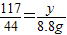 ����֮�ã�y=23.4g
����֮�ã�y=23.4g��������Һ�����ʵ�����Ϊm��
Na--NaCl
23 58.5
10.0g m
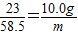 ��
����֮�ã�m=25.4g
��ԭ��Ʒ��NaCl������Ϊ��25.4g-23.4g=2.0g
ԭ��Ʒ������Ϊ=21.2+2.0=23.2g
������Һ������Ϊ��21.2g+2.0g+85.6g-8.8g=l00.0 g
��1����Ʒ��̼���Ƶ���������=
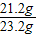 ×100%=91.4%
×100%=91.4%��2��������Һ�����ʵ���������Ϊ��
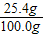 ×100%=25.4%
×100%=25.4%�𣺣�1����Ʒ��̼���Ƶ���������91.4%��
��2��������Һ�����ʵ���������Ϊ25.4%��
������������һ�����Ѷȣ���Ҫ��ԭ��Ʒ���������������õ��˹�ϵʽ�ⷨ���ݻ�ѧ����ʽ������⣬��һ�����������⣮

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ