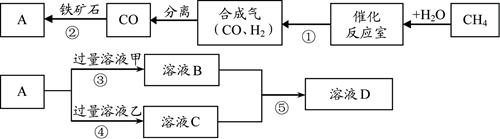
��Ŀ����
��7�֣����dz��л�ѧ�еij������ʣ���������������ش����⡣
��1�������ڳ�������Һ̬��������һЩ��Һ���ܼ�����75%����Һ����Ϊҽ����������Ļ�ѧʽΪ ��
�����ڣ�����ţ� ��
A����� B�������� C������� D�������� E��������
��2�������Ƿ��ͷ۵ijɷ�֮һ����ҽ����������θ�����֢��һ��ҩ������������� ���������ᷴӦ�Ļ�ѧ����ʽΪ ��
��3�����Ĺ�������������������������ڸ���CO2���壬��ô����Һ��pH 7���<����>�� ��=������CO2���岻�ܱ������ԭ������� ��
��4���������ʳ��ڳ��ڷ��û���ʣ�������˵����ȷ���ǣ�����ţ� ��
A����һ������������ B�����ʵ�ԭ��һ���������������˷�Ӧ
C�����ʺ�����һ������ D������һ�����ɻ�ѧ��Ӧ�����
��1�������ڳ�������Һ̬��������һЩ��Һ���ܼ�����75%����Һ����Ϊҽ����������Ļ�ѧʽΪ ��
�����ڣ�����ţ� ��
A����� B�������� C������� D�������� E��������
��2�������Ƿ��ͷ۵ijɷ�֮һ����ҽ����������θ�����֢��һ��ҩ������������� ���������ᷴӦ�Ļ�ѧ����ʽΪ ��
��3�����Ĺ�������������������������ڸ���CO2���壬��ô����Һ��pH 7���<����>�� ��=������CO2���岻�ܱ������ԭ������� ��
��4���������ʳ��ڳ��ڷ��û���ʣ�������˵����ȷ���ǣ�����ţ� ��
A����һ������������ B�����ʵ�ԭ��һ���������������˷�Ӧ
C�����ʺ�����һ������ D������һ�����ɻ�ѧ��Ӧ�����
��1��C2H5OH�� A B D
��2��С�մ�NaHCO3 + HCl NaCl + CO2�� + H2O
��3��>��CO2�����������Ӧ
��4��D
��2��С�մ�NaHCO3 + HCl NaCl + CO2�� + H2O
��3��>��CO2�����������Ӧ
��4��D
�����������1�����ڳ�������Һ̬��������һЩ��Һ���ܼ���75%����Һ����Ϊҽ������������ѧ�������ʿ�֪�������Ҵ����Ҵ���̼���⡢������Ԫ����ɣ����������л�����ڴ����Ҳ���ڻ����
��2��̼�������Ƿ��ͷ۵���Ҫ�ɷ�֮һ����ҽ����Ҳ������θ�����֢��һ��ҩ����̼�����Ƶ�������С�մ�̼�����ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ʷ�Ӧ�Ļ�ѧ����ʽΪ
NaHCO3+ HClNaCl+ CO2��+ H2O
��3���Ĺ�������������������������ڸ���CO2���壬���Լ��Ǽ��Ը�����������Һ�Լ��ԣ���pH>7��CO2���������������������ʷ�Ӧ��
��4��A�����������ڿ���Ҳ���Ա��ʣ���A����
B�������Ʊ�������ˮ��Ӧ����B����
C��˫��ˮ�������������ᣬ��C����
D�����ʾ������������������ʣ���D��ȷ����ѡD
�������ڽ������ʱ����Ҫ�Ǹ������е������ҳ�����Ҫ������ʺͷ���ʽ��Ȼ����м��鼴�ɡ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ