��Ŀ����
18��ˮ�������ǵ�����������Ƿdz���Ҫ�ģ�
��1��ͼ����ʾ���ˮ��ʵ���У��Թ�
��2��ijͬѧҪ�����ռ�������ˮ��������һ������ˮ������ͼ�ڣ�����С��ʯ��ʯӢɳ��������������

��3��ʢװ��Ȫˮ������ƿ��㡢���������Ի��������ã������ò�������
A�����ϲ��� B���л��߷��Ӳ��� C�������β��� D����������
��4������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ����
A��ϴ�˵�ˮ�������� B��ʹ�ý�ˮ��ͷ
C���ò���ϵ�ˮ����ϴ��� D��ϴ�ֲ�����ʱ������ˮ��ͷ��
��1��ͼ����ʾ���ˮ��ʵ���У��Թ�
2
���ռ�������������������2��ijͬѧҪ�����ռ�������ˮ��������һ������ˮ������ͼ�ڣ�����С��ʯ��ʯӢɳ��������������
����
��
��3��ʢװ��Ȫˮ������ƿ��㡢���������Ի��������ã������ò�������
B
��A�����ϲ��� B���л��߷��Ӳ��� C�������β��� D����������
��4������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ����
C
��A��ϴ�˵�ˮ�������� B��ʹ�ý�ˮ��ͷ
C���ò���ϵ�ˮ����ϴ��� D��ϴ�ֲ�����ʱ������ˮ��ͷ��
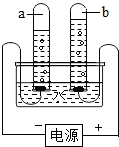
��������1�����ˮʱ���������������Թ����ɵ�����������������븺���������Թ����ɵ�����϶�����������������������������2����
��2����֪��ˮ�Ĺ��̲��ܹ����������װ�����ж����ǵ����ã�
��3�������ĺϳɲ������жϣ�
��4�������ճ������н�ˮ�Ĵ�ʩ�ͷ����������жϣ�
��2����֪��ˮ�Ĺ��̲��ܹ����������װ�����ж����ǵ����ã�
��3�������ĺϳɲ������жϣ�
��4�������ճ������н�ˮ�Ĵ�ʩ�ͷ����������жϣ�
����⣺��1�����ˮʱ���������������Թ����ɵ�����������������븺���������Թ����ɵ�����϶�����������������������������2���������Թ�2�в�����������������
��2��С��ʯ��ʯӢɳ�����������赲������ˮ�Ĺ�������ͨ����������Щ��������������ǹ��ˣ�
��3������Ϊ�ϳɲ��ϣ������л��߷��Ӳ��ϣ���ѡ��B�������⣬��ѡB��
��4��ˮ�DZ������Դ�������ǵ�����ϢϢ��أ��������ճ�������Ҫע���Լ��ˮ�����������A��B��D���ǽ�Լ��ˮ�ĺ�ϰ�ߣ�������������ѡ��C���ò���ϵ�ˮ����꣬���˷ѽ϶��ˮ�����ǽ�Լ��ˮ�ı��֣���ѡC��
�ʴ�Ϊ����1��2��
��2�����ˣ�
��3��B��
��4��C��
��2��С��ʯ��ʯӢɳ�����������赲������ˮ�Ĺ�������ͨ����������Щ��������������ǹ��ˣ�
��3������Ϊ�ϳɲ��ϣ������л��߷��Ӳ��ϣ���ѡ��B�������⣬��ѡB��
��4��ˮ�DZ������Դ�������ǵ�����ϢϢ��أ��������ճ�������Ҫע���Լ��ˮ�����������A��B��D���ǽ�Լ��ˮ�ĺ�ϰ�ߣ�������������ѡ��C���ò���ϵ�ˮ����꣬���˷ѽ϶��ˮ�����ǽ�Լ��ˮ�ı��֣���ѡC��
�ʴ�Ϊ����1��2��
��2�����ˣ�
��3��B��
��4��C��
�������˽�ˮ�ľ����������л��߷��Ӳ��ϵ����࣬��������ˮ���ʱ�������ۣ���������������������������Ϊ���������������������Ϊ2��1���ǽ������Ļ�����

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
ˮ�������ǵ�����������Ƿdz���Ҫ�ģ�
��1���ֶ�һ��ȡ�������ӵĻ��Ǻ�ˮ����������ͼ��ʾ�ľ���������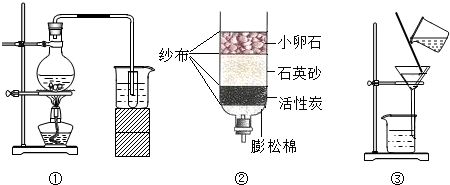
ͼ����������Һ��������������ǡ����ǡ��� ��ˮ����ͼ��ʾ�IJ����У���ˮ�ľ����̶���ߵIJ����ǣ������ƣ� ��
��2����ͼ��ʾ���ˮ��ʵ���У��Թܣ��a����b���� ���ռ���������������д����ʵ�����г�������ȡ�����Ļ�ѧ��Ӧ����ʽ ��
��3����������ҹ����ϵĺ���ǣ��������������ģ�ƿװ��Ȫˮͨ������������ԴԴ���ϵ����͵���������У�ij���Ͽ�Ȫˮƿ���ǩ����ͼ��ʾ���ÿ�Ȫˮ�к�����������������Ӫ�����е� �֣�������ƿ����������ɻ��������ã������ò��ϲ����� ��
A���ϳɲ��� B���л��߷��� C���л��� D���Ͻ�
��4������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ���� ��
A��ϴ�˵�ˮ�������� B��ʹ�ý�ˮ��ͷ
C���ò���ϵ���ˮ��ϴ��� D��ϴ�ֲ�����ʱ������ˮ��ͷ��
��1���ֶ�һ��ȡ�������ӵĻ��Ǻ�ˮ����������ͼ��ʾ�ľ���������

ͼ����������Һ��������������ǡ����ǡ���
��2����ͼ��ʾ���ˮ��ʵ���У��Թܣ��a����b����

��3����������ҹ����ϵĺ���ǣ��������������ģ�ƿװ��Ȫˮͨ������������ԴԴ���ϵ����͵���������У�ij���Ͽ�Ȫˮƿ���ǩ����ͼ��ʾ���ÿ�Ȫˮ�к�����������������Ӫ�����е�
| ÿ100mL��������g/100mL�� |
| �ơ�400 |
| þ��50 |
| �ء�35 |
| �ơ�80 |
| pH��25�棩7.3��0.5 |
��4������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ����
A��ϴ�˵�ˮ�������� B��ʹ�ý�ˮ��ͷ
C���ò���ϵ���ˮ��ϴ��� D��ϴ�ֲ�����ʱ������ˮ��ͷ��

 ��2013?�ߴ��ض�ģ����������ˮ�յ������ǣ�ˮ�������й�ˮ�ܵ������ǣ���Լ����ˮ��Դ������������̬������ˮ�������ǵ�����������Ƿdz���Ҫ�ģ�
��2013?�ߴ��ض�ģ����������ˮ�յ������ǣ�ˮ�������й�ˮ�ܵ������ǣ���Լ����ˮ��Դ������������̬������ˮ�������ǵ�����������Ƿdz���Ҫ�ģ�

