��Ŀ����
 ��2013?�ߴ��ض�ģ����������ˮ�յ������ǣ�ˮ�������й�ˮ�ܵ������ǣ���Լ����ˮ��Դ������������̬������ˮ�������ǵ�����������Ƿdz���Ҫ�ģ�
��2013?�ߴ��ض�ģ����������ˮ�յ������ǣ�ˮ�������й�ˮ�ܵ������ǣ���Լ����ˮ��Դ������������̬������ˮ�������ǵ�����������Ƿdz���Ҫ�ģ���1��ͼ����ʾ���ˮ��ʵ���У��Թ�
2
2
���ռ�������������������2��ijͬѧҪ�����ռ�������ˮ��������һ������ˮ������ͼ�ڣ�����С��ʯ��ʯӢɳ��������������
����
����
����3��ʢװ��Ȫˮ������ƿ��㡢���������Ի��������ã������ò�������
B
B
��A�����ϲ��� B���л��߷��Ӳ��� C�������β��� D����������
��4������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ����
C
C
��A��ϴ�˵�ˮ�������� B��ʹ�ý�ˮ��ͷ
C���ò���ϵ�ˮ����ϴ��� D��ϴ�ֲ�����ʱ������ˮ��ͷ
��5��ClO2����һ������ˮ����������������������Cl2��������ˮ����������ȡClO2�ķ�Ӧ����ʾ��ͼ���£�

��ش�
��C���ʵ���Է���������
58.5
58.5
����D���ʵ�������
��������
��������
���۸÷�Ӧ��A��B���ʵ���������
181��71
181��71
����������1�����ݵ��ˮ��ʵ�������������������������������Լ������������������Ϊ1��2���н��
��2������С��ʯ��ʯӢɳ�������������ǹ��˽��н��
��3���������������л��߷��Ӳ��Ͻ��н��
��4�����ݽ�Լ��ˮ�Ĵ�ʩ���н��
��5���������ʵ���Է����������ڸ�ԭ�ӵ����ԭ������֮�͡���֪ ��ʾ��ԭ�ӣ���ʾ
��ʾ��ԭ�ӣ���ʾ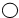 ��ʾ��ԭ�ӣ����֪D���ʵ����ơ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ��������÷�Ӧ��A��B���ʵ������Ƚ��н��
��ʾ��ԭ�ӣ����֪D���ʵ����ơ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ��������÷�Ӧ��A��B���ʵ������Ƚ��н��
��2������С��ʯ��ʯӢɳ�������������ǹ��˽��н��
��3���������������л��߷��Ӳ��Ͻ��н��
��4�����ݽ�Լ��ˮ�Ĵ�ʩ���н��
��5���������ʵ���Է����������ڸ�ԭ�ӵ����ԭ������֮�͡���֪
 ��ʾ��ԭ�ӣ���ʾ
��ʾ��ԭ�ӣ���ʾ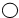 ��ʾ��ԭ�ӣ����֪D���ʵ����ơ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ��������÷�Ӧ��A��B���ʵ������Ƚ��н��
��ʾ��ԭ�ӣ����֪D���ʵ����ơ�A��B��C��D�Ļ�ѧʽ������д�÷�Ӧ�Ļ�ѧ����ʽ��������÷�Ӧ��A��B���ʵ������Ƚ��н������⣺��1�����ˮ��ʵ�������������������������������Լ������������������Ϊ1��2�������Թ�2���ռ�����������������
��2��С��ʯ��ʯӢɳ�������������ǹ��ˣ�
��3�����������л��߷��Ӳ��ϣ�
��4��A��ϴ�˵�ˮ��������������һˮ���ã����Խ�Լ��ˮ��B��ʹ�ý�ˮ��ͷ�����Խ�Լ��ˮ��C���ò���ϵ�ˮ����ϴ��꣬�˷�ˮ��Դ��D��ϴ�ֲ�����ʱ������ˮ��ͷ�����Խ�Լ��ˮ��
��5�������ʵ���Է����������ڸ�ԭ�ӵ����ԭ������֮�ͣ�C���ʵ���Է�������=23+35.5=58.5��
����֪ ��ʾ��ԭ�ӣ���ʾ
��ʾ��ԭ�ӣ���ʾ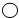 ��ʾ��ԭ�ӣ���ͼ��֪D���ʵ������Ƕ������ȣ�
��ʾ��ԭ�ӣ���ͼ��֪D���ʵ������Ƕ������ȣ�
��A��B��C��D�Ļ�ѧʽ�ֱ�Ϊ��NaClO2��Cl2��NaCl��ClO2���ʸ÷�Ӧ�Ļ�ѧ����ʽ��2NaClO2+Cl2�T2NaCl+2ClO2�����Ը÷�Ӧ��A��B���ʵ�������=��2����23+35.5+16��2��������35.5��2��=181��71��
�ʴ�Ϊ����1��2����2�����ˣ���3��B����4��C����5����58.5�� �ڶ������ȣ� ��181��71��
��2��С��ʯ��ʯӢɳ�������������ǹ��ˣ�
��3�����������л��߷��Ӳ��ϣ�
��4��A��ϴ�˵�ˮ��������������һˮ���ã����Խ�Լ��ˮ��B��ʹ�ý�ˮ��ͷ�����Խ�Լ��ˮ��C���ò���ϵ�ˮ����ϴ��꣬�˷�ˮ��Դ��D��ϴ�ֲ�����ʱ������ˮ��ͷ�����Խ�Լ��ˮ��
��5�������ʵ���Է����������ڸ�ԭ�ӵ����ԭ������֮�ͣ�C���ʵ���Է�������=23+35.5=58.5��
����֪
 ��ʾ��ԭ�ӣ���ʾ
��ʾ��ԭ�ӣ���ʾ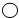 ��ʾ��ԭ�ӣ���ͼ��֪D���ʵ������Ƕ������ȣ�
��ʾ��ԭ�ӣ���ͼ��֪D���ʵ������Ƕ������ȣ���A��B��C��D�Ļ�ѧʽ�ֱ�Ϊ��NaClO2��Cl2��NaCl��ClO2���ʸ÷�Ӧ�Ļ�ѧ����ʽ��2NaClO2+Cl2�T2NaCl+2ClO2�����Ը÷�Ӧ��A��B���ʵ�������=��2����23+35.5+16��2��������35.5��2��=181��71��
�ʴ�Ϊ����1��2����2�����ˣ���3��B����4��C����5����58.5�� �ڶ������ȣ� ��181��71��
��������ˮ��Դȱ�����������صĽ��죬�˽��Լ����ˮ��Դ�Ĵ�ʩ��ˮ����Ⱦ����Դ���Ե���Ϊ��Ҫ��

��ϰ��ϵ�д�
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ
 ��2013?�ߴ��ض�ģ����ͼ�����ԭ�ӽṹʾ��ͼ����Ԫ�������ڱ��е���Ϣ������ͼ����Ϣ�жϣ�����˵����ȷ���ǣ�������
��2013?�ߴ��ض�ģ����ͼ�����ԭ�ӽṹʾ��ͼ����Ԫ�������ڱ��е���Ϣ������ͼ����Ϣ�жϣ�����˵����ȷ���ǣ�������