��Ŀ����
����Ŀ��ij����Ҫ�����̼���Ƶ������������м�⣬ֻ��������������85%���ܺϸ���ȡ15g��Ʒ�����ʲ����뷴Ӧ����98gϡ�������ʵ�飨��ӦNa2CO3+H2SO4�TNa2SO4+CO2 ��+H2O�����ֽ�ϡ�����4�μ�����Ʒ����¼����������������£�
������� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
������������ | 1.2g | 1.2g | 1.2g | 0.8g |
��1�����������������_____g
��2���ж���Ʒ�Ƿ�ϸ�д��������̣���������ȷ��0.1%��_____
���𰸡����������������4.4g ��Ʒ���ϸ�
��������
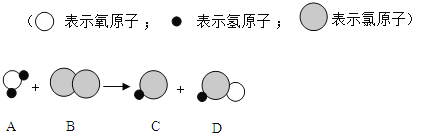
�������ɵĶ�����̼���������Ͷ�Ӧ�Ļ�ѧ����ʽ������Ʒ��̼���Ƶ���������������Աȡ�
�⣺��1������ǰ���μ�����������1.2g���壬�����Ĵ�ֻ����0.8g˵����ʱ̼�����Ѿ���ȫ��Ӧ���������ɵĶ�����̼������Ϊ1.2g��3+0.8g=4.4g��
��2������Ʒ��̼���Ƶ���������Ϊx��
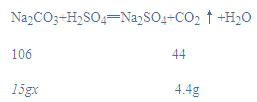
![]()
x��70.7%��85%���ϸ�

��ϰ��ϵ�д�
�����Ŀ
����Ŀ������ʵ�鷽���ܴﵽʵ��Ŀ�ĵ���
ѡ�� | A | B | C | D |
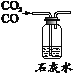
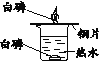
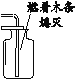
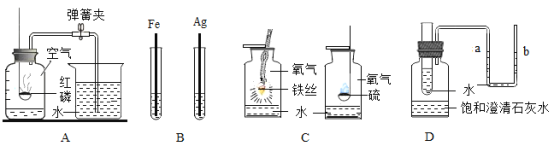
ʵ��Ŀ�� | ֤��CO2����H2O��Ӧ����H2CO3 | ��ȥCO2�е�����CO | ֤����ȼ��ȼ����Ҫ��O2�Ӵ� | ֤������ƿ���ѳ���CO2 |
ʵ�鷽�� |
|
|
|
|
A. A B. B C. C D. D