��Ŀ����
����Ŀ����ѧ��������������ϢϢ��ء�������ѧ��ѧ֪ʶ�ش��������⣺
��1�������г���_____������Ӳˮ����ˮ��
��2���˵�θҺ�ﺬ��������_____�������ƣ���������������
��3��ʳ�á��������͡���Ԥ��ƶѪ�����еġ�������ָ_____���Ԫ�ء���ԭ�ӡ�����
��4���������Ż𣬸��Ϲ��ǿ������������ԭ����_____��
��5��������ij������Ʒ���ܱ���������������O2��Ӧ��������CO2��H2O���ɴ˿�֪��������һ������_____Ԫ�ء�
��6��ϡ���С���ҵ��ά���ء���������ϡ��Ԫ��Tm��Ԫ�����ڱ��е���Ϣ��ͼ�������й�Tm���жϴ������_____������ţ���
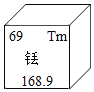
��ԭ�ӵ�������Ϊ69
�����ڽ���Ԫ��
��ԭ�ӵ�������Ϊ69
�����ԭ������Ϊ168.9
���𰸡�����ˮ ���� Ԫ�� �������� ̼���� ��
��������
��1�������г��÷���ˮ������Ӳˮ����ˮ��
��2���˵�θҺ�ﺬ�����������ᣬ������������
��3����������Ԫ����ɵģ�ʳ�á��������͡���Ԥ��ƶѪ�����еġ�������ָԪ�ء�
��4���������������Ϳ�ȼ����¶����Ż�����¡��Ƴ���ȼ�ﶼ������𡣳������Ż𣬸��Ϲ��ǿ������������ԭ���Ǹ���������
��5��������ij������Ʒ���ܱ���������������O2��Ӧ��������CO2��H2O���ɴ˿�֪��������һ������̼Ԫ�غ���Ԫ�ء�
��6������ϡ��Ԫ��Tm��Ԫ�����ڱ��е���Ϣ��֪��
��ԭ�ӵ�������Ϊ69��������ȷ��
�������ǡ������ԣ����ڽ���Ԫ�أ�������ȷ��
���������Ⱥ����������������������ϵ����������ȷ��
�����ԭ������Ϊ168.9��������ȷ����ѡ����

����Ŀ���±���KC1��NH4Cl��KNO3���������ڲ�ͬ�¶�ʱ���ܽ�ȡ�
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | KC1 | 27.6 | 34.0 | 40.0 | 45.5 | 51.1 | 56.7 |
NH4C1 | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | 77.3 | |
KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | 246 | |
���ݱ�����Ϣ��������֪��������
A. �����������ܽ����С����KCl
B. 40��ʱ��100g KNO3������Һ�к���63.9g KNO3
C. ���������У��ܽ�����¶ȱ仯Ӱ��������KNO3
D. ��0��-100�����ڵ���ͬ�¶��£�KCl��NH4Cl���ܽ�ȿ������
����Ŀ��ij��ѧ��ȤС��Ϊ̽����������ͭ�Ļ��ǿ������չ�����»��
���������ϣ�
�����ģ��������ڳ�������������е�������Ӧ�������ܵ���������Ĥ���÷�Ӧ�Ļ�ѧ����ʽΪ_____��
���Ա�ʵ�飩
��� | ���� | ���� |
�� | ������δ��ĥ����˿����CuSO4��Һ�� | ���������� |
�� | �������ĥ�����˿����CuSO4��Һ�� | ��˿����������ɫ���� |
�� | ������δ��ĥ����˿����CuCl2��Һ�� | ��˿����������ɫ���� |
��1���Ƚ�ʵ���Һ�ʵ��_____���������������������ɵ�֪����ĥ���ƻ���������Ĥ��
��2��ʵ�����з�Ӧ�Ļ�ѧ����ʽΪ_____���ݴ˿�֪�������Al��Cu_____������ǿ��������������
��3��С��ͬѧ��ʵ�����������з�������ΪH2O����������Ĥ���ƻ����á����˹۵����ϱ�����ͬѧ����������_____��
���²���̽����
С��ͬѧ���ʵ������������ۺ�²⣺Cl-�ƻ�����������Ĥ��
Ϊ����˲²��Ƿ���ȷ��������������֧�Թ��м�����ͬ��CuSO4��Һ�������������δ��ĥ����˿��Ȼ��������µ�̽����
���� | ���� | ���� | ���� |
��1����һ֧�Թ����ټ��� NaCl���� | ��˿������ ����ɫ���� | ��������Ĥ ���ƻ� | Na+���_____�� �ƻ���������Ĥ |
��2������һ֧�Թ����ټ��� Na2SO4���� | ��_____ | ��������Ĥ δ���ƻ� | Na+��SO42������ �ƻ���������Ĥ |
�������뷴˼��
�ó����ۣ�ǰ���²�_____��������ȷ����������ȷ�������ܽᷴ˼������̽����������˱ȽϷ��Ϳ��Ʊ�������