��Ŀ����
 ijʵ��С�鰴��ͼ����ľ̿��ԭ����ͭ��ʵ�飺ȡһ��������ͭ��ľ̿�Ļ�Ϸ�ĩ���ڲ��������ĸ��������·�Ӧ�������ɵ�����ͨ�����ʯ��ˮ������ʯ��ˮ����ǣ�
ijʵ��С�鰴��ͼ����ľ̿��ԭ����ͭ��ʵ�飺ȡһ��������ͭ��ľ̿�Ļ�Ϸ�ĩ���ڲ��������ĸ��������·�Ӧ�������ɵ�����ͨ�����ʯ��ˮ������ʯ��ˮ����ǣ�
�Իش�
��1�����¼��Ȼ�Ϸ�ĩʱ�۲쵽�������ǣ���ɫ��______��
��2������ʯ��ˮ�����ʱ������Ӧ�Ļ�ѧ����ʽ��______��
��3��ʵ�����ʱӦ�����ߵ��ܣ���ֹͣ���ȣ����������ȡ�������˳��ߵ����ᵼ�µĺ����______��
��4��ʵ������ľ̿��ԭ����ͭ�Ƶ�ͭ����Ҫ�Ƶ�1Ħ����ͭ��ͬʱ���ɶ�����̼�������Ƕ��ٿˣ������ݻ�ѧ����ʽ���㣬д��������̣�
�⣺��1��̼������ͭ�ڸ��������·�Ӧ����ͭ�Ͷ�����̼����˿���������Ϊ��ɫ��ĩ��ɺ�ɫ��
��2��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ��ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��3��ʵ�����������ֹͣ�����Թ���ѹǿ��С��ʯ��ˮ�ᵹ�����Թ�ʹ�Թ�ը�ѣ�
��4�������ɶ�����̼������Ϊx
C+2CuO 2Cu+CO2��
2Cu+CO2��
2 44
1mol x
 =
=
x=22g
�ʴ�Ϊ����1����ɫ��
��2��CO2+Ca��OH��2=CaCO3��+H2O��
��3��ʯ��ˮ�������Թܣ�ʹ�Թ�ը�ѣ�
��4��22g
��������1������̼������ͭ��Ӧ��������
��2�����ݶ�����̼��ʯ��ˮ��Ӧ����������ȷд����ѧ����ʽ
��3�����ݸ��������ʵ���ע��������
��4�����ݻ�ѧ����ʽ���㣮
�����������ۺϿ�����̼��ԭ����ͭʵ�飬ע��ʵ�����ʱ�ȳ����ܺƾ��ƣ�
��2��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ��ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
��3��ʵ�����������ֹͣ�����Թ���ѹǿ��С��ʯ��ˮ�ᵹ�����Թ�ʹ�Թ�ը�ѣ�
��4�������ɶ�����̼������Ϊx
C+2CuO
 2Cu+CO2��
2Cu+CO2��2 44
1mol x
 =
=
x=22g
�ʴ�Ϊ����1����ɫ��
��2��CO2+Ca��OH��2=CaCO3��+H2O��
��3��ʯ��ˮ�������Թܣ�ʹ�Թ�ը�ѣ�
��4��22g
��������1������̼������ͭ��Ӧ��������
��2�����ݶ�����̼��ʯ��ˮ��Ӧ����������ȷд����ѧ����ʽ
��3�����ݸ��������ʵ���ע��������
��4�����ݻ�ѧ����ʽ���㣮
�����������ۺϿ�����̼��ԭ����ͭʵ�飬ע��ʵ�����ʱ�ȳ����ܺƾ��ƣ�

��ϰ��ϵ�д�
�����Ŀ
ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ��� �����١��ڵ��ܿ�����ʱ��B�п�����ʵ�������ǣ� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
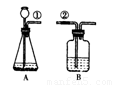
�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
|
ʵ�� |
��1�� |
��2�� |
��3�� |
��4�� |
|
������Ʒ������/g |
5 |
10 |
15 |
20 |
|
����CO2������/g |
1.54 |
3.08 |
4.4 |
m |
���ݱ���������������㣺
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
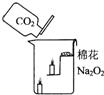 22���������ƣ�Na2O2����һ��dz��ɫ���壮ij�οƼ���У�ij��ѧ��ȤС���ͬѧ������Na2O2������������ձ��У�����ͼ������������CO2���������������Ϩ������ĺ�Ϩ��ͬʱҲ����ķ�����ȼ�������ˣ�
22���������ƣ�Na2O2����һ��dz��ɫ���壮ij�οƼ���У�ij��ѧ��ȤС���ͬѧ������Na2O2������������ձ��У�����ͼ������������CO2���������������Ϩ������ĺ�Ϩ��ͬʱҲ����ķ�����ȼ�������ˣ�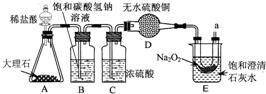
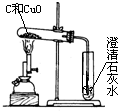 ��2010?��ɽ��һģ��ijʵ��С�鰴��ͼ����ľ̿��ԭ����ͭ��ʵ�飺ȡһ��������ͭ��ľ̿�Ļ�Ϸ�ĩ���ڲ��������ĸ��������·�Ӧ�������ɵ�����ͨ�����ʯ��ˮ������ʯ��ˮ����ǣ�
��2010?��ɽ��һģ��ijʵ��С�鰴��ͼ����ľ̿��ԭ����ͭ��ʵ�飺ȡһ��������ͭ��ľ̿�Ļ�Ϸ�ĩ���ڲ��������ĸ��������·�Ӧ�������ɵ�����ͨ�����ʯ��ˮ������ʯ��ˮ����ǣ�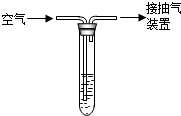 ����������ڿ�����ȼ������SO2��SO2��һ����ɫ���д̼�����ζ���ж����壮
����������ڿ�����ȼ������SO2��SO2��һ����ɫ���д̼�����ζ���ж����壮