��Ŀ����
ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ��� �����١��ڵ��ܿ�����ʱ��B�п�����ʵ�������ǣ� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
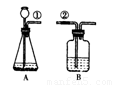
�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
|
ʵ�� |
��1�� |
��2�� |
��3�� |
��4�� |
|
������Ʒ������/g |
5 |
10 |
15 |
20 |
|
����CO2������/g |
1.54 |
3.08 |
4.4 |
m |
���ݱ���������������㣺
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
ʯ����Һ ʯ����Һ����ɫ���ɫ CO2 + H2O === H2 CO3
��ɫ����ʧ�ֱ���ɫ H2 CO3 === H2 O + CO2��
��1����һ������ ��2��4.4
ע������ÿ�ո�1�֣���7��
��3���⣺�� ����1.54g CO2 ��Ҫ̼�������Ϊx��0.5�֣�
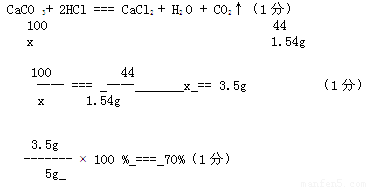
���ԣ�0.5�֣��������ⷨ��������Ҳ���֣�
����������

��1����������
�ٴ����к��н϶�Ŀ��������ʣ�MgCl2��CaCl2�ȣ��Ͳ��������ʣ���ɳ�ȣ���
���Ȼ��������£�20�棩ʱ���ܽ��Ϊ36.0g��
��Mg2+��Ca2+�ļ���ε��ܽ��ԣ�20�棩���±���
| ������\������ | OH- | NO3- | SO42- | CO32- |
| Mg2+ | �� | �� | �� | |
| Ca2+ | | �� | | �� |
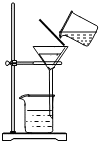
��2��ʵ�鷽���������������
���ܽ⣨20�棩��������ƽ��ȡ10.0g���Σ���ҩ���ô������뵽ʢ��10mLˮ���ձ���ӱ߽��裬ֱ�����β����ܽ�Ϊֹ����ʱ����Ĵ�������������
�ڳ�����Ҫ��ȥ�����еĿ���������CaCl2���������м��������Na2CO3��Һ����Ҫ��ȥ����MgCl2���������м��������
������
��3��ʵ��ʵ����������ԣ�
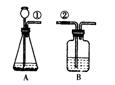
ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ��� �����١��ڵ��ܿ�����ʱ��B�п�����ʵ�������ǣ� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
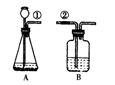
�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
| ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1.54 | 3.08 | 4.4 | m |
���ݱ���������������㣺
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
| ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1.54 | 3.08 | 4.4 | m |
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ��� �����١��ڵ��ܿ�����ʱ��B�п�����ʵ�������ǣ� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
�����ܶϿ�һ��ʱ�����B�е������� ��
�䷴Ӧ�Ļ�ѧ����ʽΪ�� .
Ϊ�ⶨ����ʯ��ʯ��̼��Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
| ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1.54 | 3.08 | 4.4 | m |
��1���ļ��Ӧ��������ʣ�� ��
��2���ϱ���m����ֵ�� ��
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������