��Ŀ����
ijѧУ��ѧϰС��Ե��ص�ʯ��ʯ�������е��飬�ⶨʯ��ʯ��̼��Ƶ��������������õķ������£����ɼ�������Ʒ��ˮ��ϴ�����ɣ���ȡ20.00g��Ʒƽ���ֳ����ݣ��ֱ���������ͬ����������ϡ���ᷴӦ�������вⶨ����ͼ1���������ݴ����õ��ͷų�������̼�������뷴Ӧʱ��Ĺ�ϵͼ����ͼ2����
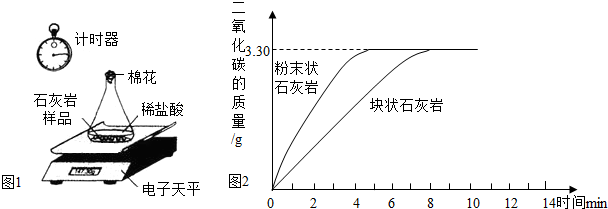
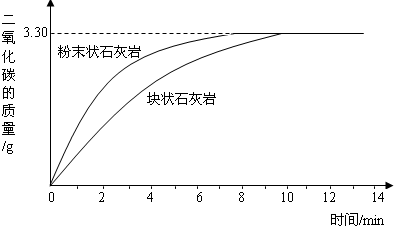
��1����ͼ2�����߿��Կ���������������Һ�����ʷ�Ӧ��������������ͬʱ���Ӵ����Խ ���䷴Ӧ����Խ ��
��2��������Ʒ��̼��Ƶ�����������������Ʒ���������ʲ��μӷ�Ӧ��������ˮ���Ȼ����ݳ�����

��1����ͼ2�����߿��Կ���������������Һ�����ʷ�Ӧ��������������ͬʱ���Ӵ����Խ
��2��������Ʒ��̼��Ƶ�����������������Ʒ���������ʲ��μӷ�Ӧ��������ˮ���Ȼ����ݳ�����
����������������ͼ����������м������Ŀ����ͼ����Կ�����ĩ״̼�����ȫ��Ӧʱ�ȿ�״��Ӧ�����ʱ��̣�����Ӧ�ٶȿ죻
��Ʒ�е�̼������������ɸ������ɵĶ�����̼�������������ʯ������̼��Ƶ���������⣮
��Ʒ�е�̼������������ɸ������ɵĶ�����̼�������������ʯ������̼��Ƶ���������⣮
����⣺��1����ͼ����Կ�����ĩ״̼�����ȫ��Ӧʱ�ȿ�״��Ӧ�����ʱ��̣�����Ӧ�ٶȿ죬Ҳ���ǽӴ������ʱ��Ӧ�ٶȿ죮
��2����ͼ���֪�÷�Ӧ���ɵĶ�����̼������3.3g
��10g��Ʒ�к�̼�������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.3g
=
x=7.5g
��100%=75%
�ʴ�Ϊ����1����С�����죨����������2������Ʒ��̼��Ƶ�����������75%��
��2����ͼ���֪�÷�Ӧ���ɵĶ�����̼������3.3g
��10g��Ʒ�к�̼�������Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.3g
| 100 |
| x |
| 44 |
| 3.3g |
x=7.5g
| 7.5g |
| 10g |
�ʴ�Ϊ����1����С�����죨����������2������Ʒ��̼��Ƶ�����������75%��
����������������ͼ�鷴Ӧ�ٶȵ�Ӱ�����أ���ͨ��ͼ������˸��ݻ�ѧ����ʽ�ļ��㣬������ѧ���ķ��������������������

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
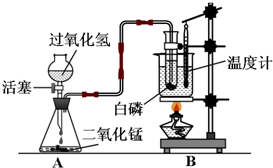 ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮
ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮 ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮
ijѧУ��ѧѧϰС����Ƴ���ͼ��ʾװ�ã������а���ȼ��ʵ�飮