��Ŀ����
 ˮ������֮Դ��������������Դ����������Ҫ�й���ˮ������ˮ����Լˮ����ʶ���������������ش����⣺
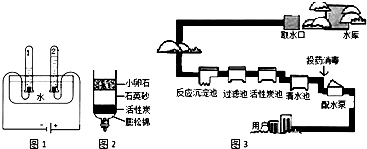
ˮ������֮Դ��������������Դ����������Ҫ�й���ˮ������ˮ����Լˮ����ʶ���������������ش����⣺��1��ͼ1�ǵ��ˮ��ʵ��װ�ã�ͨ��һ��ʱ���������������������Ϊ
��2��ͼ2�Ǽ���װ�ã���װ����С��ʯ��ʯӢɰ��������
��3��ͼ3������ˮ����ˮ����ʾ��ͼ������ˮ����������ˮʱ��ʹ�õľ�ˮ������
A������ B������ C����� D������ E������
��4��С��ͬѧ������Լҵľ�ˮ��Ӳˮ������ˮ������
��3��������˿ȼ�յ�ʵ��ʱ������ƿ��ʢ����ˮ��������
��4���������ȣ�ClO2������һ������ˮ������������ҵ����ȡ�������ȵķ����ǣ���������Cl2��ͨ���������ƣ�NaClO2����Һ�з�Ӧ�����ɶ������Ⱥ��Ȼ��ƣ�д���÷�Ӧ�Ļ�ѧ����ʽ
��5�������������һ��ˮ������������ᡱ����仰��ʾ����Ҫ��������ˮ��Դ����ʶ��һ�ǽ�Լ��ˮ�����Ƿ�ֹˮ����Ⱦ�����һ����Լ��ˮ��������
���㣺���ˮʵ��,�����Ļ�ѧ����,����ˮ�����������뾻������,Ӳˮ����ˮ,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ,����ˮ��Դ�ͽ�Լ��ˮ
ר�⣺������ˮ
��������1���ɵ��ˮ��ʵ��װ��ͼ����ֻ�Թ������������ϵ�����ݵ��ˮʱ���������������Ϊ2��1��ȷ���������������Թ��ڵ����壬�����ˮ���Ļ�ѧ����ʽ��
��2�����ݼ���ˮװ��ͼ��С��ʯ��ʯӢɰ���Ѳ�����ˮ�����ʳ�ȥ���ݴ˷�����ɣ�
��3����������ˮ����������ˮʱ���õľ�ˮ�������з����жϣ�
��4������Ӳˮ����ˮ���õķ������÷���ˮ�������ճ���������Ӳˮ�ķ������н��
��3����������������ȼ�յ�ʵ��������з������
��4�����ݶԷ�Ӧ�����������ȷ���μӷ�Ӧ�����ʼ����ɵ����ʣ���ɷ�Ӧ��ѧ����ʽ����д��
��5�����ݽ�Լ��ˮ�ķ������з������
��2�����ݼ���ˮװ��ͼ��С��ʯ��ʯӢɰ���Ѳ�����ˮ�����ʳ�ȥ���ݴ˷�����ɣ�
��3����������ˮ����������ˮʱ���õľ�ˮ�������з����жϣ�
��4������Ӳˮ����ˮ���õķ������÷���ˮ�������ճ���������Ӳˮ�ķ������н��
��3����������������ȼ�յ�ʵ��������з������
��4�����ݶԷ�Ӧ�����������ȷ���μӷ�Ӧ�����ʼ����ɵ����ʣ���ɷ�Ӧ��ѧ����ʽ����д��
��5�����ݽ�Լ��ˮ�ķ������з������
����⣺��1��ͼʾ���Դ�����������Թ�������Լ�븺���Թ������������һ�룬���жϸ��Թ�����������Ϊ������ˮ��ͨ�������¿ɷֽ�������������������Ӧ�Ļ�ѧ����ʽΪ2H2O
2H2��+O2����
��2�����ݾ�ˮ����װ��ͼ����֪ˮ����С��ʯ�ɳ�ȥ�ϴ�����Ĺ��岻�����ʯӢɰ��ɳ�ȥС�����Ĺ��岻������С��ʯ��ʯӢɰ�����˵����ã�������װ�õ�ˮ�����ܼ������иơ�þ���ӣ���˲��ܽ�Ӳˮ������
��3������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�����
��4��Ӳˮ����ˮ���������������ĸ�þ���ӵĶ��٣������п��÷���ˮ������Ӳˮ����ˮ��������ĭ�϶������ˮ�����ٵ�Ӳˮ�������г�����л�����ķ���������ˮ��Ӳ�ȣ�
��3��������˿ȼ�յ�ʵ��ʱ������ƿ��ʢ����ˮ��Ϊ�˷�ֹ�������ۻ�����������ʹƿ��ը�ѣ�
��4������������Һ�����������Ʒ�Ӧ���ɶ������Ⱥ��Ȼ��ƿ�֪���û�ѧ����ʽΪCl2 +2NaClO2 =2NaCl+2ClO2��
��5����Լ��ˮ����������ˢ���ÿڱ���ˮ����ҵ��ˮ�ظ����ã�ϴ��ˮ�����ϵغ��ٳ�������ֲ�����ཽˮ��������ˮ���д����������õȣ�
�ʴ�Ϊ����1��������2H2O
2H2��+O2������2�����ˣ����ܣ� ��3��ABE����4������ˮ����У���3����ֹ�������ۻ�����������ʹƿ��ը�ѣ���4��Cl2+2NaClO2=2NaCl+2ClO2����5��ˢ���ÿڱ���ˮ����ҵ��ˮ�ظ����ã�ϴ��ˮ�����ϵغ��ٳ�������ֲ�����ཽˮ��������ˮ���д����������õȣ�
| ||
��2�����ݾ�ˮ����װ��ͼ����֪ˮ����С��ʯ�ɳ�ȥ�ϴ�����Ĺ��岻�����ʯӢɰ��ɳ�ȥС�����Ĺ��岻������С��ʯ��ʯӢɰ�����˵����ã�������װ�õ�ˮ�����ܼ������иơ�þ���ӣ���˲��ܽ�Ӳˮ������
��3������ˮ����������ˮʱ��ʹ�õľ�ˮ�����г��������ˡ�����
��4��Ӳˮ����ˮ���������������ĸ�þ���ӵĶ��٣������п��÷���ˮ������Ӳˮ����ˮ��������ĭ�϶������ˮ�����ٵ�Ӳˮ�������г�����л�����ķ���������ˮ��Ӳ�ȣ�
��3��������˿ȼ�յ�ʵ��ʱ������ƿ��ʢ����ˮ��Ϊ�˷�ֹ�������ۻ�����������ʹƿ��ը�ѣ�
��4������������Һ�����������Ʒ�Ӧ���ɶ������Ⱥ��Ȼ��ƿ�֪���û�ѧ����ʽΪCl2 +2NaClO2 =2NaCl+2ClO2��
��5����Լ��ˮ����������ˢ���ÿڱ���ˮ����ҵ��ˮ�ظ����ã�ϴ��ˮ�����ϵغ��ٳ�������ֲ�����ཽˮ��������ˮ���д����������õȣ�
�ʴ�Ϊ����1��������2H2O
| ||
�����������ѶȲ�����������ˮ������ˮ�ķ�����Ӳˮ����ˮ��������ת����������Լ��ˮ�ķ���������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ


 ��ͼ�Ǽ��ö������������ͼʾ�ش��������⣺
��ͼ�Ǽ��ö������������ͼʾ�ش��������⣺