��Ŀ����
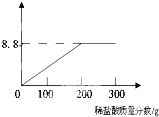 ���ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3����26.5g������69.3��ˮ�������Ƴ���Һ���������м���һ������ϡ���ᣬʹ������ȫ�ų����������������������꣩�����������������ͼ��ʾ��ע�⣺�����NaCl��Ӧ��ͼ�е�����������λ���ǿˣ���
���ҹ��ຣ��������һ��˵���������̼����ɹ�Σ�����ļ���ָNa2CO3������ָNaCl�����Ǵ��κ����̵õ�Na2CO3�Ậ��������NaCl��ij�о���ѧϰС���ȡ��NaCl��Na2CO3����26.5g������69.3��ˮ�������Ƴ���Һ���������м���һ������ϡ���ᣬʹ������ȫ�ų����������������������꣩�����������������ͼ��ʾ��ע�⣺�����NaCl��Ӧ��ͼ�е�����������λ���ǿˣ������㣺
��1��ԭ������Na2CO3������������
��2�������������������������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1��̼���������ᷴӦ�ų�������̼���Ȼ��Ʋ������ᷴӦ�����ݷ�Ӧ�Ļ�ѧ����ʽ�������ɶ�����̼�������ɼ�����Ʒ��̼���Ƶ�������̼���Ƶ���������Ʒ�����ıȿɼ���ԭ������Na2CO3������������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�������ɶ�����̼�������ɼ���������������������ͼ������������������������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�������ɶ�����̼�������ɼ���������������������ͼ������������������������
����⣺
����Ʒ��̼���Ƶ�����Ϊx�����ɶ�����̼8.8g������HCl������Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y 8.8g
=
x=21.2g
=
y=14.6g
��1��ԭ������Na2CO3����������=
��100%=80%
��2�����������������������
��100%=7.3%
�𰸣�
��1��ԭ������Na2CO3����������80%
��2�����������������������7.3%
����Ʒ��̼���Ƶ�����Ϊx�����ɶ�����̼8.8g������HCl������Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y 8.8g
| 106 |
| 44 |
| x |
| 8.8g |
x=21.2g
| 73 |
| 44 |
| y |
| 8.8g |
y=14.6g
��1��ԭ������Na2CO3����������=
| 21.2g |
| 26.5g |
��2�����������������������
| 14.6g |
| 200g |
�𰸣�
��1��ԭ������Na2CO3����������80%
��2�����������������������7.3%
���������ݻ�ѧ����ʽ���Ա�ʾ��Ӧ�и����ʵ������ȣ��ɷ�Ӧ���������ʵ������ɼ������Ӧ���������ʵ�������

��ϰ��ϵ�д�
�����Ŀ
���м��ֳ�����ʳ���У�ά���غ�����ḻ���ǣ�������
A�� ������ |
B�� ���� |
C�� ƻ�� |
D�� �� |
���������У��������ˮ����Ⱦ���ǣ�������
| A����ũ��������������ũҩ |
| B��������ˮֱ���ŷŵ������� |
| C����ˮ�з���������Ϻ |
| D��ʹ�ú���ϴ�·� |

 ����ͼ��ʾ̽�������˶���ʵ���У�
����ͼ��ʾ̽�������˶���ʵ���У�